Antiaromatic cyclic compounds, which have 4n π-electrons, are often unstable, and thus, difficult to synthesize. Replacing carbon units in aromatic compounds with metal-based fragments is one method for tuning their aromaticity. Naphthalene, for example, is a fused-ring aromatic compound that could be used in such an approach.
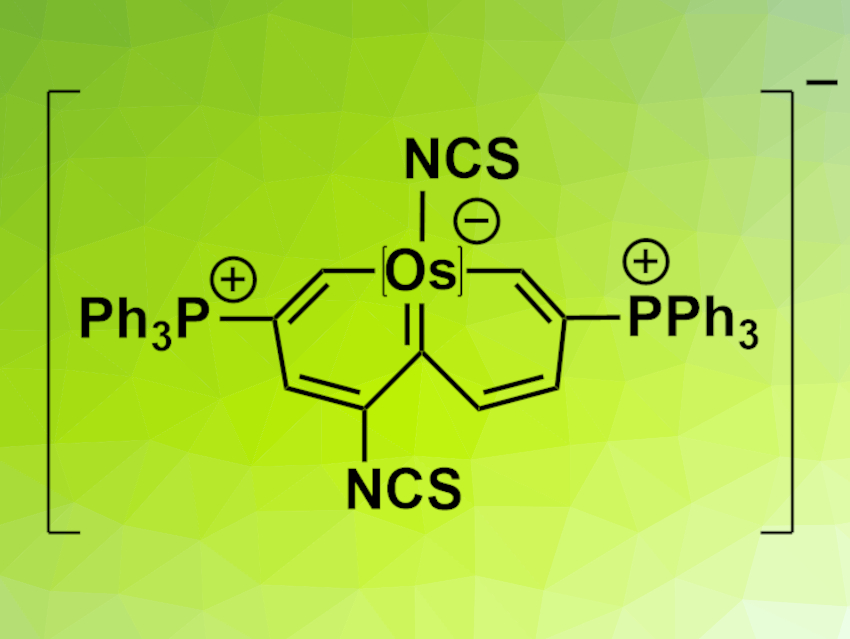
Jun Zhu, Haiping Xia, Xiamen University, China, and colleagues have synthesized naphthalene derivatives with a metal-based bridgehead fragment (example pictured), i.e., osmanaphthalenes. They started from a linear precursor ligand with the required nine carbon atoms to form the osmanaphthalenes’ carbon framework and reacted it with OsCl2(PPh3)3. The resulting ten-membered osmacycle was reacted with dichlorodicyanobenzoquinone (DDQ) and NH4Cl to give a chloro-functionalized osmanaphthalene. This compound was unstable in solution. However, replacing the Cl atoms using NaSCN and NaPF6 gave a complex stable enough for characterization using X-ray diffraction (pictured).
The team found that the osmanaphthalene has a highly twisted conformation. The researchers propose that this is due to a higher stability of the twisted structure compared with an analogous planar one—the planar structure would be antiaromatic, as calculations indicate.
- Releasing Antiaromaticity in Metal-Bridgehead Naphthalene,
Chun Tang, Yu Zhao, Jingjing Wu, Zhixin Chen, Liu Leo Liu, Yuan-Zhi Tan, Jun Zhu, Haiping Xia,
J. Am. Chem. Soc. 2021.
https://doi.org/10.1021/jacs.1c08106