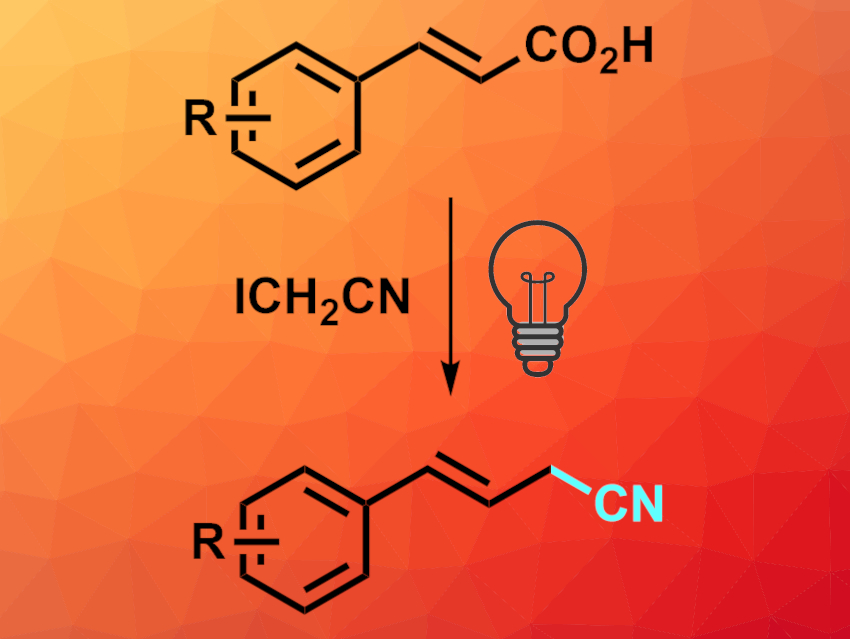
Nitriles are useful intermediates in organic synthesis. β,γ-Unsaturated nitriles, in particular, are interesting precursors in synthetic chemistry and can show biological activities. Efficient methods for their synthesis are, thus, useful.
Yongyun Zhou, Baomin Fan, Yunnan Minzu University, Kunming, China, and colleagues have developed a metal- and photocatalyst-free, low-cost, environmentally friendly decarboxylative alkylation of α,β-unsaturated carboxylic acids to give β,γ-unsaturated nitriles (pictured). The team reacted a variety of α,β-unsaturated carboxylic acids, e.g., cinnamic acid derivatives, with 2-iodoacetonitrile (ICH2CN) in the presence of K2CO3 and water under blue light-emitting diode (LED) irradiation in tetrahydrofuran (THF) as a solvent at room temperature.
The desired β,γ-unsaturated nitriles were obtained in moderate to good yields. The reaction can be performed on a gram scale. The products can be further transformed, e.g., by hydrogenation or epoxidation of the C=C bond, conversion of the CN group to an ester, or hydrolysis to give a carboxylic acid.
- Photo-Promoted Decarboxylative Alkylation of α,β-Unsaturated Carboxylic Acids with ICH2CN for the Synthesis of β,γ-Unsaturated Nitriles,
Chunxiang Pan, Chunhui Yang, Kangkui Li, Keyang Zhang, Yuanbin Zhu, Shiyuan Wu, Yongyun Zhou, Baomin Fan,
Org. Lett. 2021.
https://doi.org/10.1021/acs.orglett.1c02585