Electronic displays, solar cells, or catalysts often contain rare metals. For example, metal-to-ligand charge transfer (MLCT) excited states of Ru(II) and Ir(III) complexes can play key roles in, e.g., light-emitting devices, dye-sensitized solar cells, photoredox catalysis, artificial photosynthesis, or photodynamic therapy. These rare metals are expensive and can be toxic. Low-cost, more abundant alternatives based on elements such as manganese would be useful.
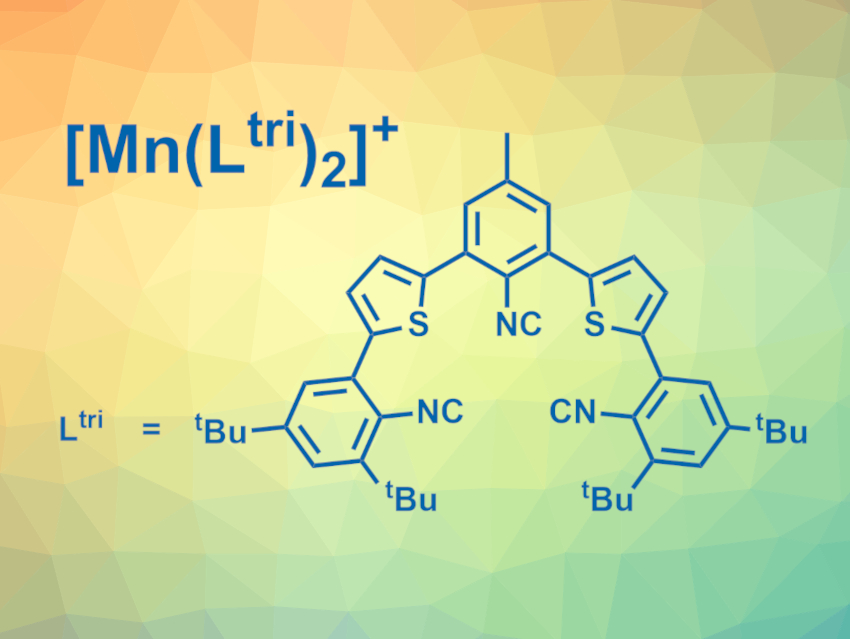
Oliver S. Wenger, University of Basel, Switzerland, and colleagues have developed air-stable manganese(I) complexes with isocyanide chelate ligands (example pictured) that show MLCT luminescence in solution at room temperature. The team used two different isocyanide chelate ligands with thiophene units in the backbone, one of them bidentate and one tridentate (Ltri, pictured). The ligands were reacted with Mn(CO)5Br to obtain the complexes [Mn(Lbi)3]+ and [Mn(Ltri)2]+.
According to the researchers, the products represent the first air-stable complexes of an element in a 3d6 configuration with luminescent MLCT states, and photoactive excited states of the complexes are sufficiently long-lived for bimolecular reactions. The team successfully used the complexes as photosensitizers and to promote the photoisomerization of trans-stilbene.
- Manganese(I) complexes with metal-to-ligand charge transfer luminescence and photoreactivity,
Patrick Herr, Christoph Kerzig, Christopher B. Larsen, Daniel Häussinger, Oliver S. Wenger,
Nat. Chem. 2021.
https://doi.org/10.1038/s41557-021-00744-9