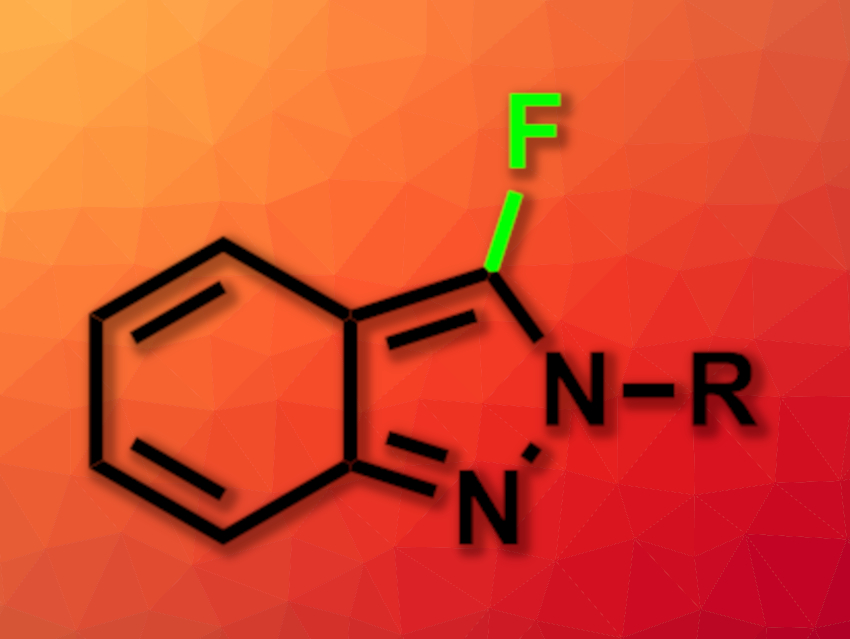
Fluorinated organic compounds are useful, e.g., in pharmaceutical chemistry. Fluorine atoms can be introduced using reagents such as tetrabutylammoniumfluoride (TBAF), Selectfluor, or N-fluorobenzenesulfonimide (NFSI). The latter is particularly convenient because it is a non-hygroscopic, stable, easy-to-handle solid.
Payel Ghosh and Alakananda Hajra, Visva-Bharati University, Santiniketan, India, have developed a simple, metal-free fluorination of 2H-indazoles (product pictured) in water using NFSI as a fluorine source. The team used various 2H-indazoles as substrates and reacted them with NFSI in water at 80 °C, with reaction times ranging from 15 min to 8 h, depending on the substrate. A range of 2-phenyl-2H-indazole derivatives, 2-benzyl-2H-indazole, and a 2H-indazole with a tert-butyl group at the N-2 position were converted successfully.
The desired fluorinated products were obtained in mostly good yields and with good regioselectively for the C-3 position. The protocol uses water as an environmentally friendly solvent and can be performed on a gram scale.
- Fluorination of 2H-Indazoles Using N-Fluorobenzenesulfonimide,
Payel Ghosh, Alakananda Hajra,
J. Org. Chem. 2021.
https://doi.org/10.1021/acs.joc.1c01253