Cages such as fullerenes, as well as similar saturated species such as hydrogenated fullerenes (fulleranes) or edge-fused oligoadamantanes (diamondoids) are interesting subjects in nanochemistry. Much less is known about analogues of these compounds with the heavier silicon instead of carbon. There are, e.g., no examples of silicon-based fullerene analogues in the condensed phase. Saturated silafulleranes such as siladodecahedrane (Si20H20) could be more stable, and thus, promising targets for synthesis.
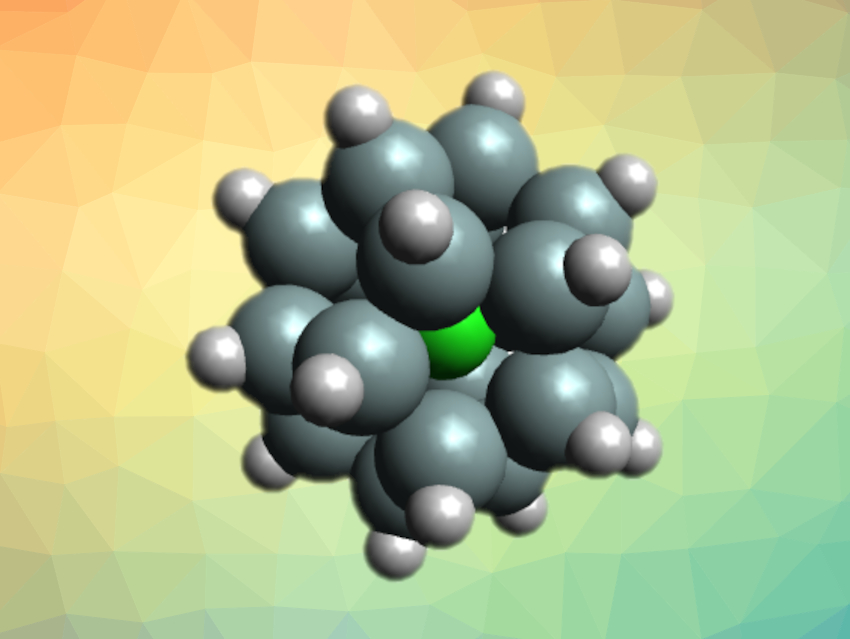
Stefan Grimme, University of Bonn, Germany, Matthias Wagner, University of Frankfurt am Main, Germany, and colleagues have synthesized siladodecahedrane with an encapsulated chloride ion, [Cl@Si20H20]− (pictured). The team started from [nBu4N][Cl@Si32Cl44], which contains a Si20 dodecahedron. This precursor was desilylated via a reaction with pinacol to give [Cl@Si20H12Cl8]−. Then, a hydrogenation reaction with iBu2AlH gave the desired siladodecahedrane.
The team found that [Cl@Si20H20]− can be perchlorinated by treating it with CDCl3 to give [Cl@Si20Cl20]−. They characterized both of these compounds using X-ray diffraction, mass spectrometry (MS), as well as 1H, 29Si, and 35Cl NMR spectroscopy and confirmed their highly symmetric structures.
- [Cl@Si20H20]−: Parent Siladodecahedrane with Endohedral Chloride Ion,
Marcel Bamberg, Markus Bursch, Andreas Hansen, Matthias Brandl, Gabriele Sentis, Lukas Kunze, Michael Bolte, Hans-Wolfram Lerner, Stefan Grimme, Matthias Wagner,
J. Am. Chem. Soc. 2021.
https://doi.org/10.1021/jacs.1c05598