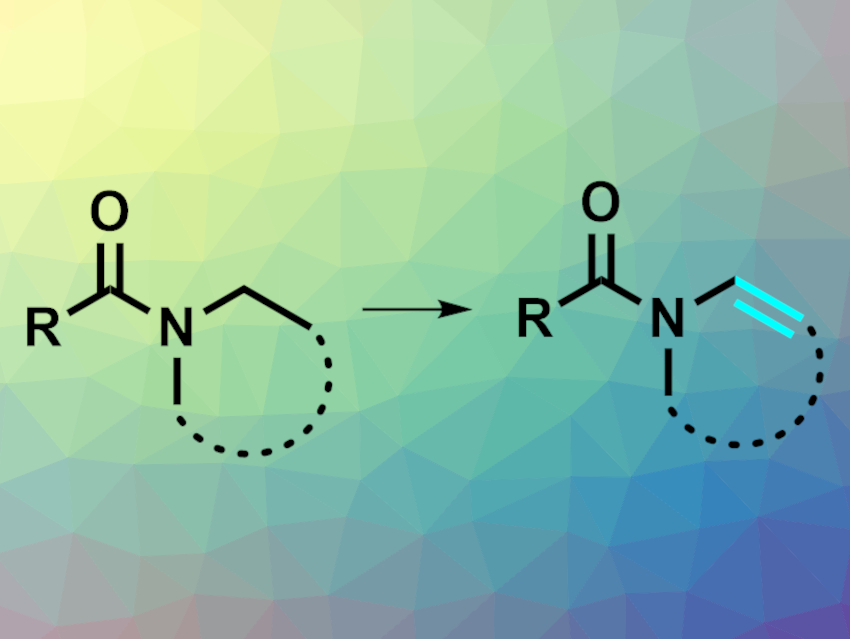
Enamines are useful building blocks in organic synthesis. They can be synthesized from prefunctionalized substrates, but a direct dehydrogenation of the corresponding amides (pictured) could be a simpler approach. Amides can be activated to allow for a variety of reactions, but such reactions are usually limited to the carbonyl subunit, while much less is known about the N-functionalization of amides.
Nuno Maulide, University of Vienna, Austria, and colleagues have developed a reaction protocol that allows the transformation of amides to enamides via electrophilic amide activation. As substrates, the team used a variety of amides with different aromatic substituents on the carbonyl side and cyclic or acyclic groups on the nitrogen side. These were reacted with a combination of lithium hexamethyldisilazide (LiHMDS) and triflic anhydride (Tf2O) in diethyl ether as a solvent at –94 °C. The reagents act as both the oxidant and as an electrophilic activator for the amide. The team proposes the formation of an activated iminium triflate intermediate.
The desired enamines were obtained in mostly good yields. The products can be used in a wide range of further functionalization reactions. According to the researchers, this is the first general one-step approach for the synthesis of N-cyclic and -acyclic enamides that does not require prefunctionalized substrates.
- Direct Synthesis of Enamides via Electrophilic Activation of Amides,
Philipp Spieß, Martin Berger, Daniel Kaiser, Nuno Maulide,
J. Am. Chem. Soc. 2021.
https://doi.org/10.1021/jacs.1c04363