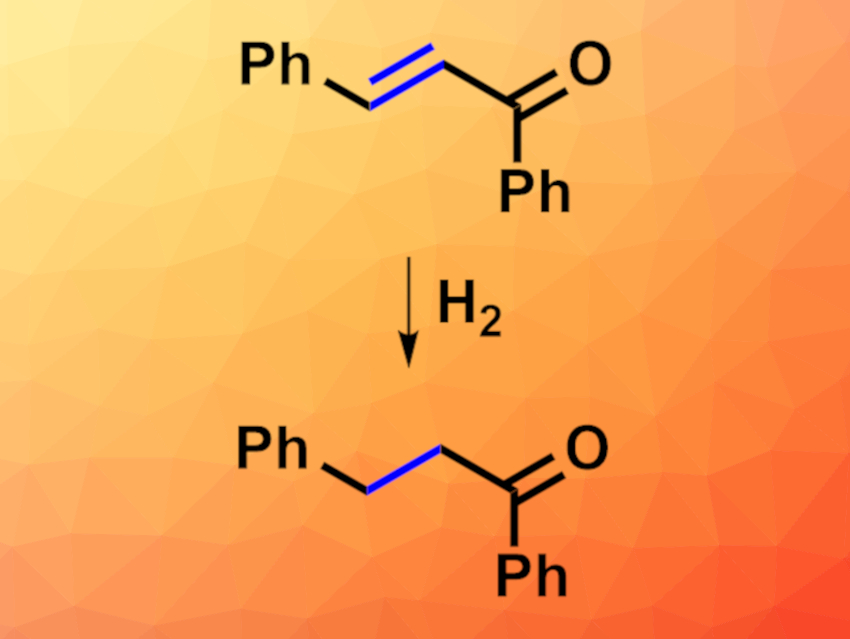
The catalytic hydrogenation of C=C bonds is widely used in organic synthesis. The selective hydrogenation of α,β-unsaturated carbonyl compounds, for example, can be very useful, but challenging for some substrates due to steric hindrance or insufficient functional group tolerance.
Jack R. Norton, Columbia University, New York City, NY, USA, and colleagues have found that the rhodium hydride (η5-C5Me5)Rh(2-(2-pyridyl)phenyl)H catalyzes the selective hydrogenation of the C=C double bond in α,β-unsaturated carbonyl compounds (example pictured), as well as the hydrogenation of many isolated C=C bonds. Using this catalyst, the team reacted a variety of α,β-unsaturated carbonyl compounds such as enones and α,β-unsaturated esters with H2, with methanol as the solvent. The reactions were performed at room temperature. The approach can also be used for the hydrogenation of a range of isolated olefins.
The reaction formed the desired C=C-hydrogenation products with high chemoselectivities and in good to excellent yields. Substrates with bulky substituents can be converted, and a variety of functional groups are tolerated. According to the researchers, the approach may also be useful in late-stage reductions, e.g., during the synthesis of pharmaceutically active compounds.
- Highly Selective Hydrogenation of C=C Bonds Catalyzed by a Rhodium Hydride,
Yiting Gu, Jack R. Norton, Farbod Salahi, Vladislav G. Lisnyak, Zhiyao Zhou, Scott A. Snyder,
J. Am. Chem. Soc. 2021.
https://doi.org/10.1021/jacs.1c04683