Mechanical forces can drive chemical changes in controlled and useful ways. This can be achieved by incorporating mechanophores into a material, i.e., molecular units that are sensitive to mechanical stress or strain. Mechanochromic units, for example, can change their optical properties in response to mechanical stimuli. However, the response mechanism of most mechanophores involves breaking chemical bonds. Thus, they need relatively large mechanical forces to be activated and their response is usually not reversible.
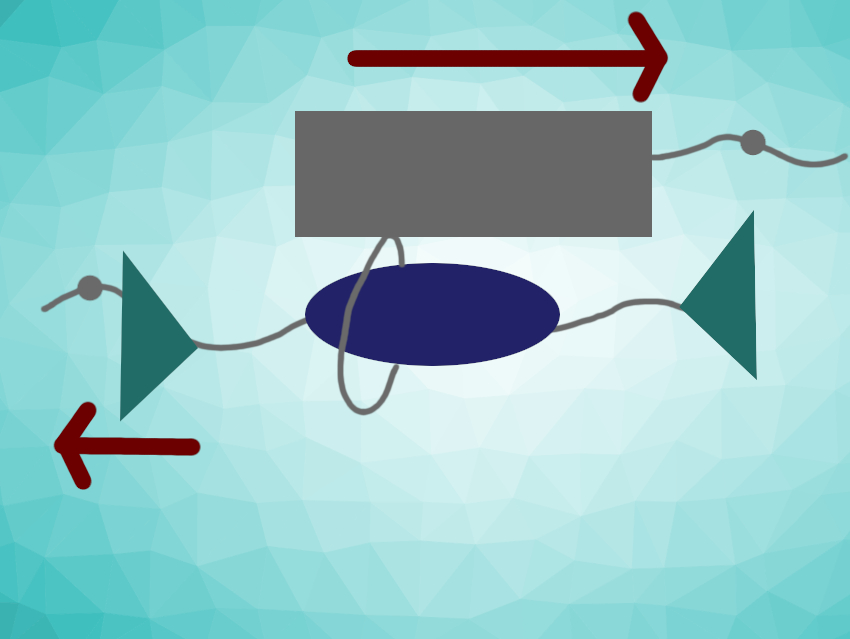
Christoph Weder, University of Fribourg, Switzerland, Yoshimitsu Sagara, Tokyo Institute of Technology, Japan, and colleagues have developed rotaxane-based supramolecular mechanophores that show both reversible and irreversible fluorescence changes triggered by mechanical forces. The rotaxanes are composed of a ring attached to a luminophore (pictured in grey), which is threaded onto an axle with a matching quencher (pictured in blue) and two stoppers (pictured in green). The compounds were synthesized via 1,3-dipolar cycloaddition click reactions between the axle precursors in the presence of the ring.
When no force is exerted on the rotaxanes, the luminophore is close to the quencher and its emission is quenched. When a force is applied (see arrows), the luminophore slides along the axle away from the quencher and its fluorescence is “turned on”. This process is reversible. However, when a particularly large force is used, the ring can slip over the stopper and be “dethreaded”, leading to an irreversible fluorescence change. In principle, such rotaxanes could be used to monitor the current strain in a polymer material as well as visualize mechanical damage that was inflicted in the past and induced irreversible changes.
- Rotaxane-Based Dual Function Mechanophores Exhibiting Reversible and Irreversible Responses,
Tatsuya Muramatsu, Yuji Okado, Hanna Traeger, Stephen Schrettl, Nobuyuki Tamaoki, Christoph Weder, Yoshimitsu Sagara,
J. Am. Chem. Soc. 2021.
https://doi.org/10.1021/jacs.1c03790