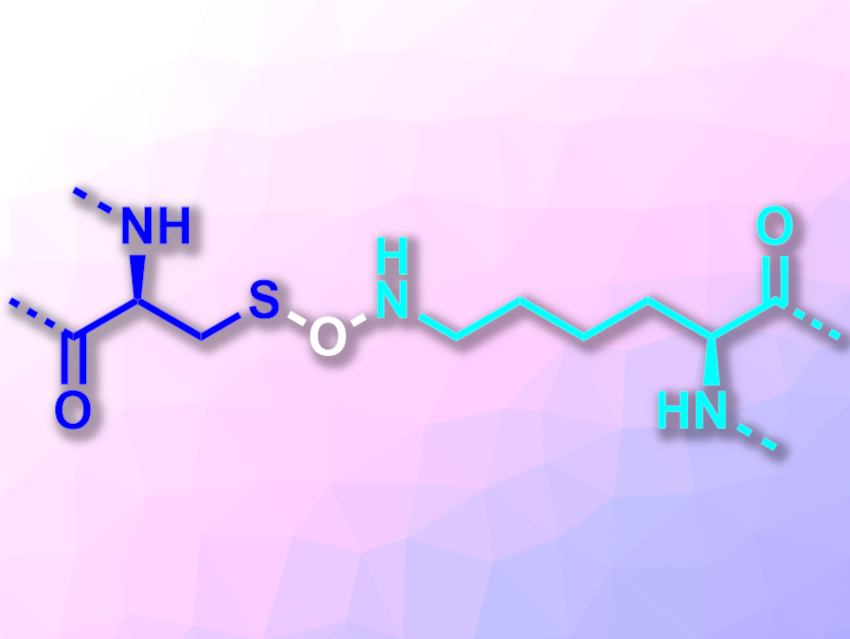
In proteins, disulfide bridges can form between cysteine residues and stabilize the overall three-dimensional structure. In contrast to most other interactions between protein chains, these bridges are formed by covalent bonds. Disulfide bridges can be reversibly formed and broken by redox reactions. This means they can be used as “redox switches” to influence protein structure and function.
Kai Tittmann, University of Göttingen and Max Planck Institute for Biophysical Chemistry, Göttingen, Germany, and colleagues have found a second type of cysteine-based redox switch with a covalent crosslink in proteins: an N–O–S bridge formed between a cysteine and a lysine residue (pictured, cysteine on the left, lysine on the right). The team investigated a transaldolase enzyme from the human pathogen Neisseria gonorrhoeae, which causes gonorrhea. This enzyme’s activity can be “switched” on or off by reducing or oxidizing it, respectively. The researchers crystallized this protein under either oxidizing or reducing conditions and recorded X-ray structures.
In the oxidized state of the protein, the team found a crosslink between a cysteine and a lysine residue with a bridging oxygen atom. In the reduced state, this covalent N–O–S bridge was absent. According to the researchers, the N–O–S bridge is not unique to the studied transaldolase, but might also exist in other proteins. They searched the Protein Data Bank (PDB) for suitable structures and identified several proteins that are likely to possess this type of bridge.
- A lysine–cysteine redox switch with an NOS bridge regulates enzyme function,
Marie Wensien, Fabian Rabe von Pappenheim, Lisa-Marie Funk, Patrick Kloskowski, Ute Curth, Ulf Diederichsen, Jon Uranga, Jin Ye, Pan Fang, Kuan-Ting Pan, Henning Urlaub, Ricardo A. Mata, Viktor Sautner, Kai Tittmann,
Nature 2021.
https://doi.org/10.1038/s41586-021-03513-3