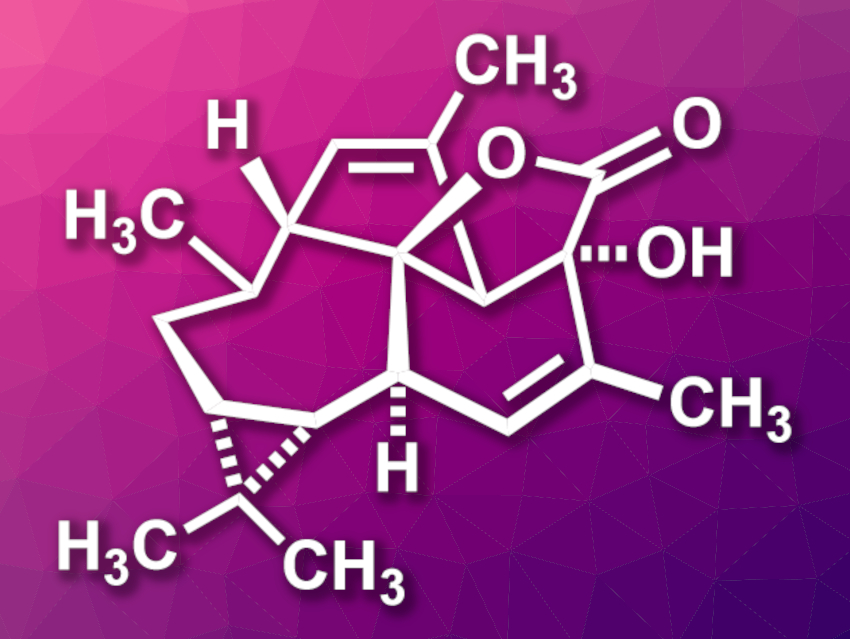
Euphorikanin A (pictured) is a natural product that was isolated from a plant in the spurge family. The compound showed cytotoxic activity against human cancer cell lines. Euphorikanin A has a complex structure with a 5/6/7/3-fused tetracyclic skeleton and eight stereocenters.
Erick M. Carreira, Swiss Federal Institute of Technology (ETH) Zurich, Switzerland, and colleagues have performed the first total synthesis of (+)-euphorikanin A. The team started from (+)-3-carene, a compound with fused cyclopropane and cyclohexene rings, which was converted to a cycloheptenone in four steps. The cycloheptenone intermediate was reacted with Me2CuLi and an aldehyde to introduce an alcohol-functionalized side chain. This was followed by a one-pot sequence of reactions, concluding with the conversion of the primary side-chain alcohol into an aldehyde.
A Still–Gennari olefination of the aldehyde intermediate gave a Z-enoate, which served as the precursor for a key SmI2-mediated ketyl–enoate reaction that was used to form two rings in a single step. In the final steps, the C=C bond was introduced into the five-membered ring and the cyclohexene ring of the product was formed. Overall, (+)-euphorikanin A was prepared in 19 steps from (+)-3-carene.
- Enantioselective Total Synthesis of (+)-Euphorikanin A,
Moritz J. Classen, Markus N. A. Böcker, Remo Roth, Willi M. Amberg, Erick M. Carreira,
J. Am. Chem. Soc. 2021.
https://doi.org/10.1021/jacs.1c04210