In organic synthesis, the central carbon skeleton is most often prepared in the early stages, while the later stages focus on transformations around the periphery of the molecules and C–H functionalizations. “Editing” the skeleton of a compound in later stages could be useful, but strategies that can achieve this are limited.
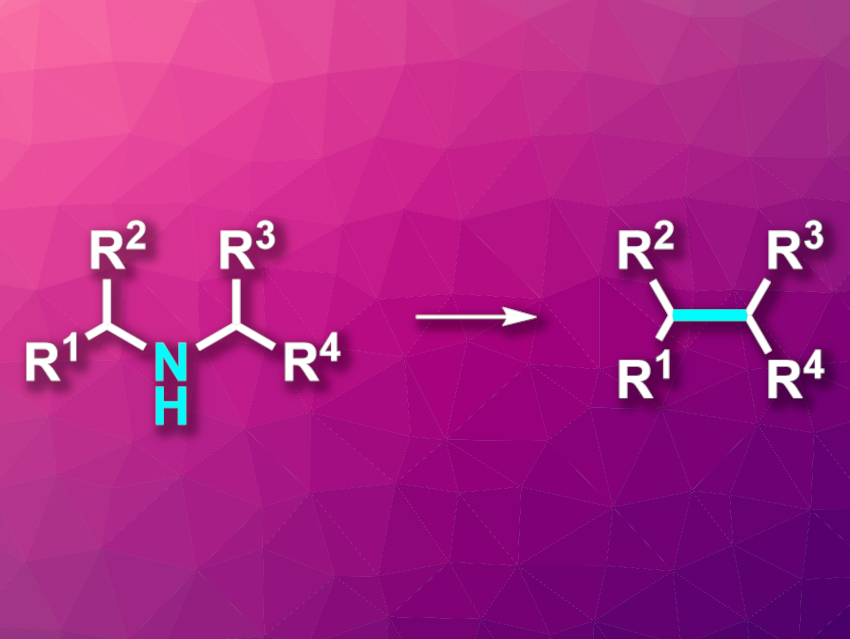
Mark D. Levin, University of Chicago, IL, USA, and colleagues have developed a reaction that “cuts” nitrogen from secondary aliphatic amines, transforming them to intramolecular C–C coupling products (pictured). The team used a variety of acyclic and cyclic secondary amines as substrates, which were reacted with N-(benzyloxy)-N-(pivaloyloxy)-4-(trifluoromethyl)benzamide—an anomeric amide (i.e., an amide with two oxygen substituents at the amide nitrogen). The reactions were performed at 45 °C in tetrahydrofuran (THF).
The desired “nitrogen-deletion” products were obtained in moderate to good yields. The researchers propose a mechanism that starts with a nucleophilic substitution of the pivaloyloxy group in the reagent by the amine substrate to release pivalic acid. This is followed by a reductive elimination that releases a benzoate ester and gives an isodiazene intermediate of the type RR’N–N. Loss of an N2 molecule then generates a pair of carbon radicals, which combine to form a C–C bond in the place of the original amine. This method provides an alternative way to synthesize carbon–carbon coupling products via an amine intermediate.
- Skeletal editing through direct nitrogen deletion of secondary amines,
Sean H. Kennedy, Balu D. Dherange, Kathleen J. Berger, Mark D. Levin,
Nature 2021.
https://doi.org/10.1038/s41586-021-03448-9