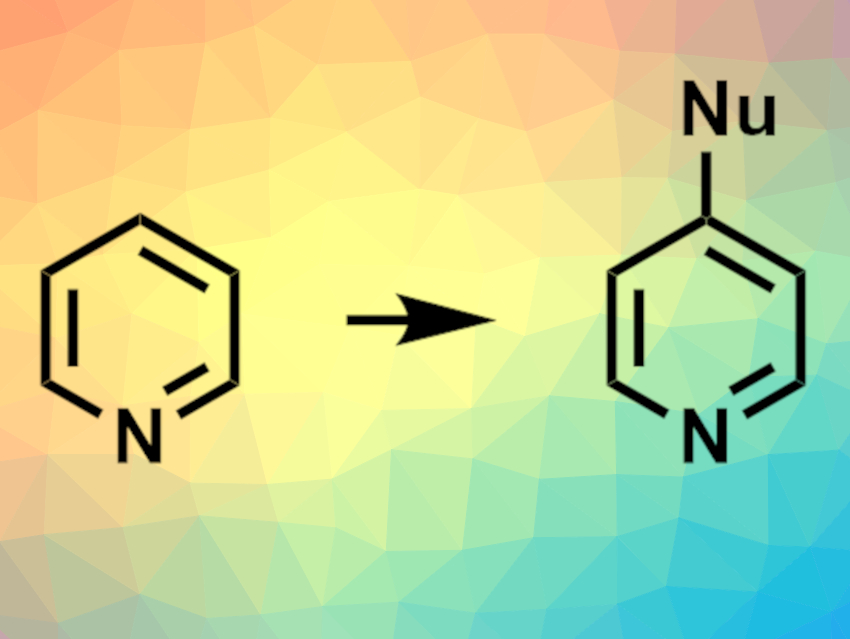
Nitrogen-containing heterocycles are found, e.g., in many pharmaceutically active compounds. Methods for functionalizing N-heterocycles are, thus, important for drug discovery. Late-stage modifications that can be performed under mild conditions and tolerate a wide range of functional groups are particularly useful in this context. Silylium ions, the Si equivalents of carbenium ions, can be used as Lewis-acid catalysts in organic synthesis. However, they had not been used to activate six-membered N-heterocycles (azines) for C–C bond-forming reactions so far.
Carla Obradors and Benjamin List, Max Planck Institute for Coal Research, Mülheim an der Ruhr, Germany, have developed a method for the functionalization of azines via silylium activation. The team used a phosphoramidimidate derivative (PhPADI, pictured on the right) as a Brønsted acid precatalyst, a variety of silyl ketene acetals as reagents, and pyridines, pyridazines, pyrimidines, quinolines, and quinazolines as the  N-heterocyclic substrates. Acetonitrile was used as the solvent and 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) as a reagent for oxidative quenching. The reactions were performed at 25 °C in a one-pot setup.
N-heterocyclic substrates. Acetonitrile was used as the solvent and 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) as a reagent for oxidative quenching. The reactions were performed at 25 °C in a one-pot setup.
The team obtained the desired functionalized azines in high to excellent yields and with high regioselectivity. The reaction tolerates a wide variety of functional groups. The researchers propose a mechanism that involves the generation of a silylated species from the precatalyst. The silylium unit then coordinates to the substrate and activates it. Finally, a reaction with the silyl ketene acetal gives the desired product and the silylium species is released again.
- Azine Activation via Silylium Catalysis,
Carla Obradors, Benjamin List,
J. Am. Chem. Soc. 2021.
https://doi.org/10.1021/jacs.1c03257