Metallabenzenes, i.e., benzene derivatives that include one or more transition-metal atoms in the ring, are usually studied for their unusual structures, bonding properties, and aromaticity. They have also been proposed as intermediates in some transition-metal-catalyzed reactions, e.g., tungstenabenzenes in alkyne polymerization reactions. However, metallabenzenes have generally not been used as catalysts themselves.
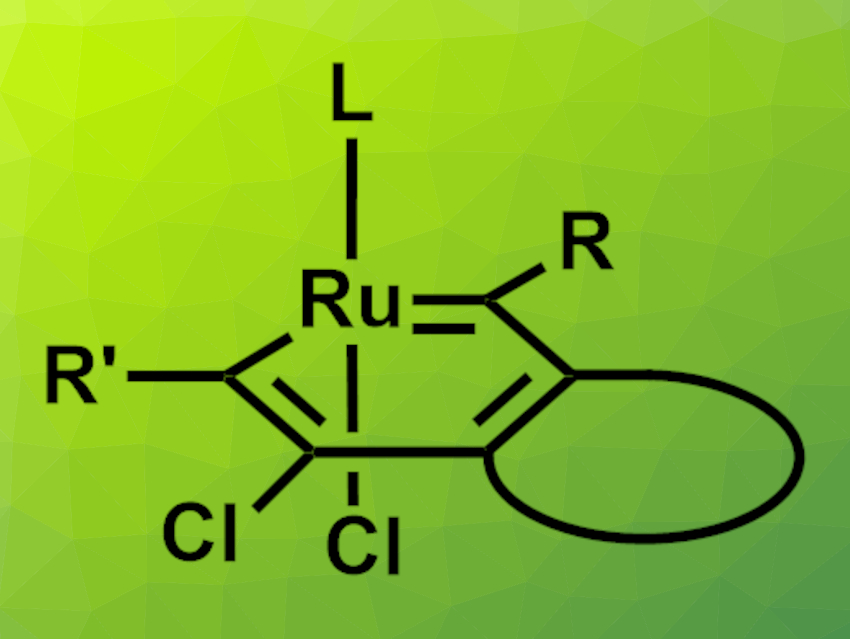
Peng Liu, University of Pittsburgh, PA, USA, Daesung Lee, University of Illinois at Chicago, USA, and colleagues have synthesized different ruthenabenzenes (general structure pictured above) and used them as catalysts for metathesis reactions. The team first prepared triynes, which were reacted with a Grubbs-Hoveyda catalyst (example pictured on the right) to give alkyne-chelated ruthenium alkylidene intermediates. These intermediates then spontaneously reorganize into the desired ruthenabenzenes, driven by the aromatic stabilization in the resulting rings.
to give alkyne-chelated ruthenium alkylidene intermediates. These intermediates then spontaneously reorganize into the desired ruthenabenzenes, driven by the aromatic stabilization in the resulting rings.
The team proposed that the ruthenabenzes could act as aromatic equivalents of Grubbs-type ruthenium alkylidene catalysts that are commonly used in olefin metathesis. They evaluated the catalytic activity of the synthesized ruthenabenzenes in ring-closing metathesis (RCM) reactions. They found that the ruthenabenzenes can act as precatalysts, i.e., they release small amounts of ring-opened, alkyne-chelated ruthenium alkylidene species that then catalyze the RCM reaction. This was supported by the results of density-functional theory (DFT) calculations. According to the researchers, the work shows that metallabenzenes could be useful platforms for catalyst development.
- Ruthenabenzene: A Robust Precatalyst,
Saswata Gupta, Siyuan Su, Yu Zhang, Peng Liu, Donald J. Wink, Daesung Lee,
J. Am. Chem. Soc. 2021.
https://doi.org/10.1021/jacs.1c02237