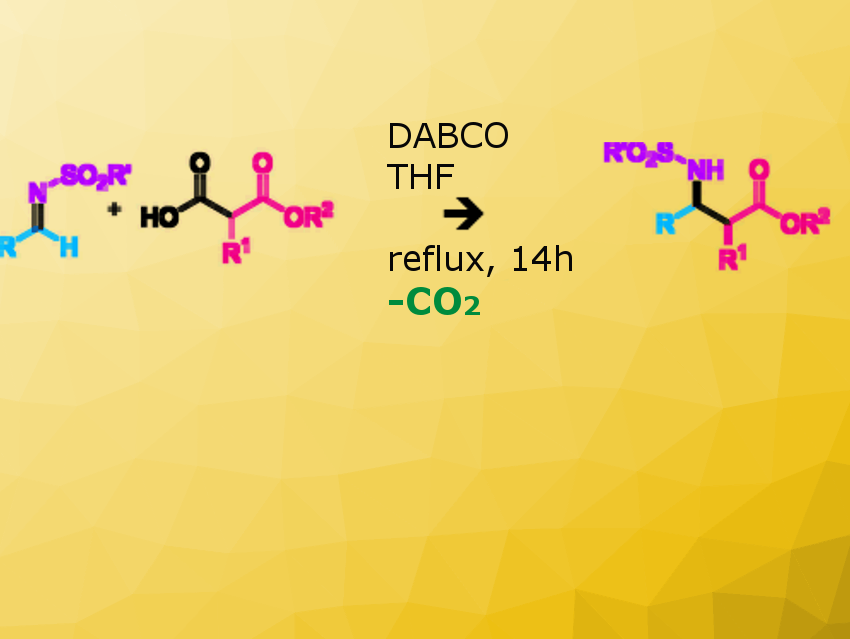
Malonic acid half-oxyesters (MAHOs) and malonic acid half-thioesters (MAHTs) can be converted to ester or thioester enolates, respectively, by decarboxylation under mild reaction conditions, avoiding the use of either strong bases or Lewis acids and thus increasing the environmental compatibility of the transformation.
Sylvie Condon, Marc Presset, and colleagues, Université Paris Est Créteil, CNRS, ICMPE, Thiais, France, have developed a new route for the preparation of β2,3-aminoesters involving an organocatalyzed decarboxylative Mannich reaction. The team began by the optimization of the decarboxylative Mannich reaction between tosyl imines and substituted malonic acids half-oxyesters (SMAHOs).
The tertiary amine, 1,4-diazabicyclo[2.2.2]octane (DABCO), was used as catalyst, and tetrahydrofuran (THF) as solvent. The reaction proceeds under simple reaction conditions and tolerates a broad range of substrates, affording general access to β<sup>2,3</sup>-aminoesters, the syn diastereomer being the major one. An alternative multicomponent protocol has also been developed to increase the overall eco-compatibility of the process.
- Decarboxylative Mannich Reactions with Substituted Malonic Acid Half-Oxyesters,
Tania Xavier, Sylvie Condon, Christophe Pichon, Erwan Le Gall, Marc Presset,
J. Org. Chem. 2021.
https://doi.org/10.1021/acs.joc.0c02895