Carboranes are clusters composed of boron, carbon, and hydrogen atoms. In ortho– or o-carboranes, two neighboring vertices of the cluster cage are C–H units. These clusters can be considered three-dimensional versions of benzene derivatives. Just like benzene can be converted to the reactive benzyne by formally removing two hydrogen atoms, the ortho-carborane o-C2B10H12 can be converted to 1,2-dehydro-o-carborane, or o-carboryne.
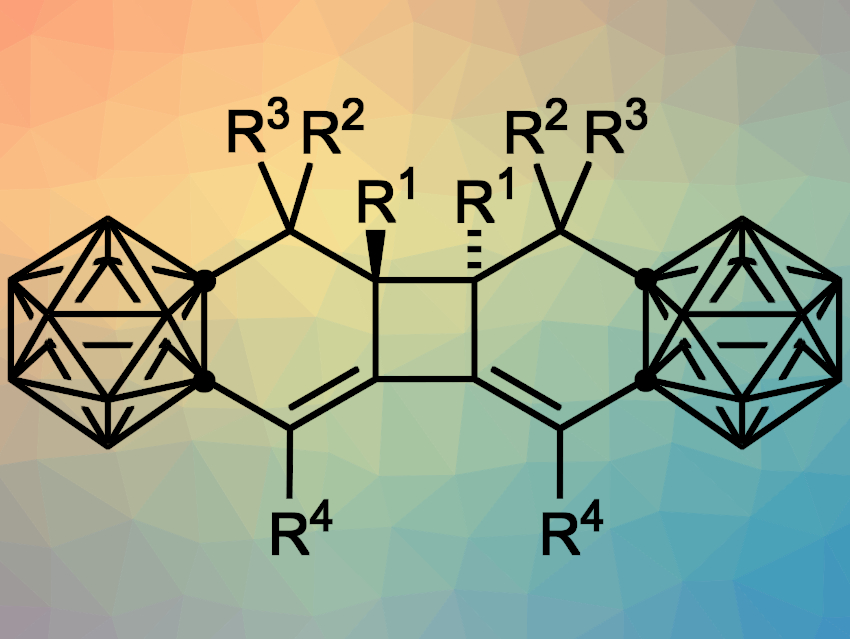
Jie Zhang and Zuowei Xie, The Chinese University of Hong Kong, China, have used o-carboryne for [4+2] cycloadditions with conjugated enynes, followed by a [2+2] cycloaddition to give carborane-fused tricyclic compounds (pictured, tricyclo[6.4.0.02,7]dodeca-2,12-dienes). The team used 1-Li-2-OTf-o-C2B10H10 as a carboryne precursor, which was prepared in situ from 1-OTf-o-C2B10H11 and lithium bis(trimethylsilyl)amide (LiHMDS). This was directly followed by a tandem cycloaddition reaction with conjugated enynes, which was performed in cyclohexane at room temperature.
The desired carborane-fused tricyclic compounds were obtained in moderate to good yields. According to the researchers, the reaction first generates a reactive carborane-fused 1,2-cyclohexadiene intermediate, which then undergoes a [2+2] cycloaddition to form the central four-membered ring.
- Tandem [4 + 2]/[2 + 2] cycloaddition of o-carboryne with enynes: facile construction of carborane-fused tricyclics,
Jie Zhang, Zuowei Xie,
Chem. Sci. 2021.
https://doi.org/10.1039/d0sc07047e