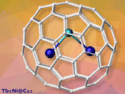
Carboranes are clusters composed of boron, carbon, and hydrogen atoms. In ortho-carboranes, two neighboring vertices of the cluster cage are C–H units. They are robust and could be useful for a variety of applications. However, their stability can make functionalization reactions difficult.
Yangjian Quan, Zuowei Xie, The Chinese University of Hong Kong, China, and colleagues have developed a reaction for the selective B–H amination of ortho-carboranes using ammonia. The reaction is based on an iridium-catalyzed B–H/N–H dehydrocoupling, i.e., a reaction that formally eliminates H2 from one B–H bond of the carborane and one N–H bond of ammonia. It selectively aminates a boron atom adjacent to the two C–H vertices (product pictured). The team used [Ir(cod)OMe]2 (cod = 1,5-cyclooctadiene) as a catalyst, PCy3 as a ligand (Cy = cyclohexyl), K2CO3 as a base, and tetrahydrofuran (THF) as the solvent to aminate a variety of C- or B-substituted ortho-carboranes using NH3.
The desired products were obtained in moderate to good yields. The reaction uses cheap ammonia gas as a reactant and produces H2 as the only byproduct. According to the researchers, the work provides a new pathway for the direct, efficient, and regioselective B–H amination of ortho-carboranes.
- Ir-Catalyzed Selective B(3)-H Amination of o-Carboranes with NH3,
Yik Ki Au, Jie Zhang, Yangjian Quan, Zuowei Xie,
J. Am. Chem. Soc. 2021.
https://doi.org/10.1021/jacs.1c00593