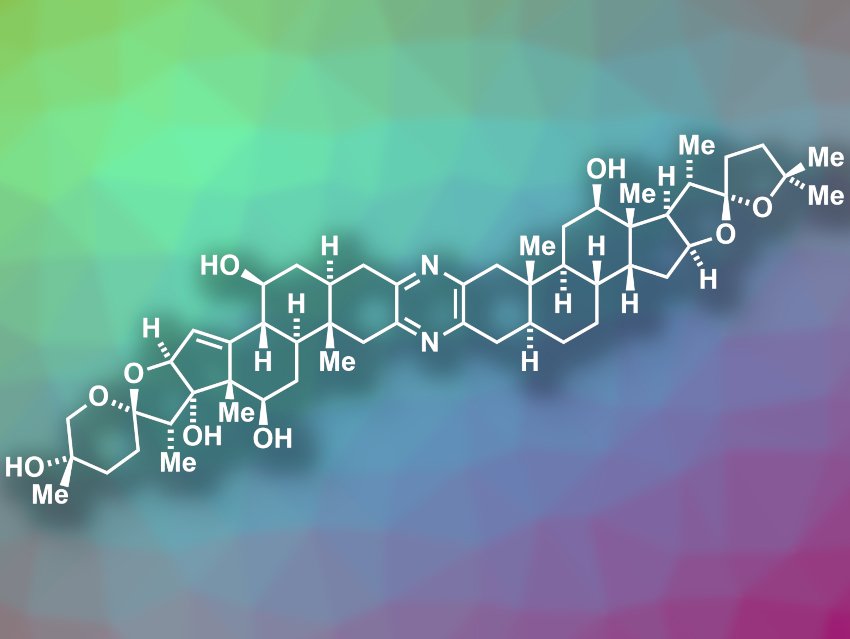
Ritterazine B (pictured) is a natural product with a bis-steroidal pyrazine structure. It was isolated from Riterella tokioka, a marine invertebrate. The compound has shown potent anticancer activity, e.g., against leukemia cells. However, the amount of natural material of ritterazine B is small, which limits investigations of its biological and medicinal properties.
Sarah E. Reisman, California Institute of Technology (Caltech), Pasadena, USA, and colleagues have performed the first total synthesis of ritterazine B. The team used a synthetic strategy that starts from the commercially available steroid trans-dehydroandrosterone. This compound was converted to an α-hydroxyketone-functionalized steroid that is used as a common intermediate to form the two different steroid fragments of the final product. This intermediate was reacted with different propargyl bromides to obtain alkyne-functionalized steroid precursors of the two “halves” of ritterazine B. The alkyne side chains were then converted to the two different spiroketals situated at the ends of the final product.
The synthesis was completed by the heterodimerization of the two different spiroketal-containing, steroid-based units to form the central pyrazine, followed by global deprotection. According to the researchers, the synthesis provides sufficient amounts of product for further investigations of the biological activity of ritterazine B.
- Total Synthesis of Ritterazine B,
Yasuaki Nakayama, Michael R. Maser, Tatsuya Okita, Anton V. Dubrovskiy, Taryn L. Campbell, Sarah E. Reisman,
J. Am. Chem. Soc. 2021.
https://doi.org/10.1021/jacs.1c01372