Simple, oxygen-containing organic molecules in interstellar space could be created by reactions of small carbon clusters with water. The simplest of these compounds have the formula C3H2O. Isomers with this formula such as propynal, cyclopropenone, or propadienone have been detected in space or under cryogenic laboratory conditions.
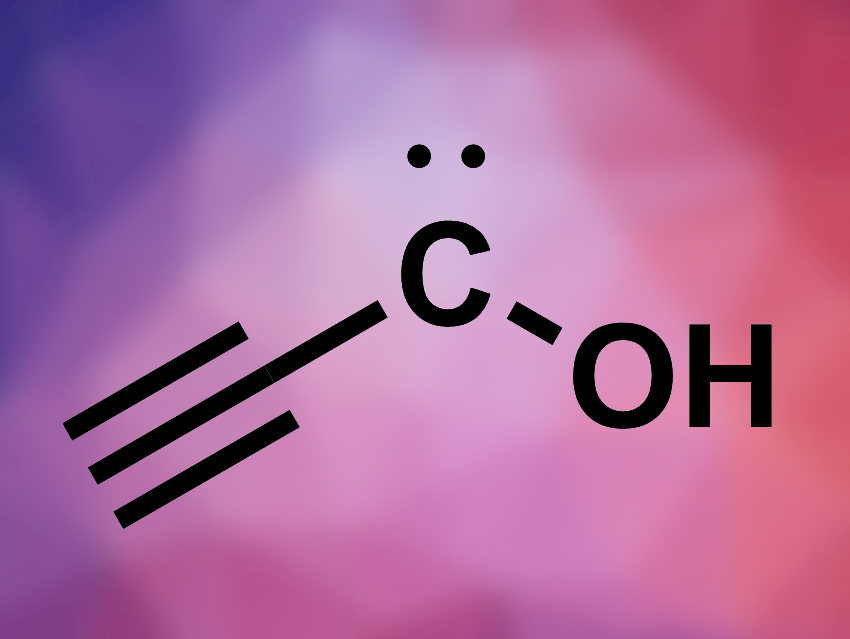
Peter R. Schreiner, University of Giessen, Germany, and colleagues have characterized a so far unreported C3H2O isomer, ethynylhydroxycarbene (HC≡C–C̈–OH, pictured). The team first synthesized ethynylglyoxylic acid ethyl ester (HC≡C–C(O)–CO2Et) from 2-hydroxybut-3-ynoic acid ethyl ester using Dess-Martin periodinane. This precursor was then pyrolyzed at 900 °C and the pyrolysis products were condensed in a solid argon matrix at 3–20 K.
The researchers used infrared (IR) spectroscopy to characterize the pyrolysis products and compared the spectra with simulated spectra from density functional theory (DFT) calculations. The IR spectra indicated the presence of ethynylhydroxycarbene. The team also investigated the reactivity of ethynylhydroxycarbene. They found that irradiation at 436 nm first leads to the conversion of its trans-conformer to the cis-conformer and then to the formation of propynal. In the dark, ethynylhydroxycarbene is converted to propynal via a proton-tunneling process.
- Ethynylhydroxycarbene (H–C≡C–C̈–OH),
Bastian Bernhardt, Marcel Ruth, André K. Eckhardt, Peter R. Schreiner,
J. Am. Chem. Soc. 2021.
https://doi.org/10.1021/jacs.1c00897