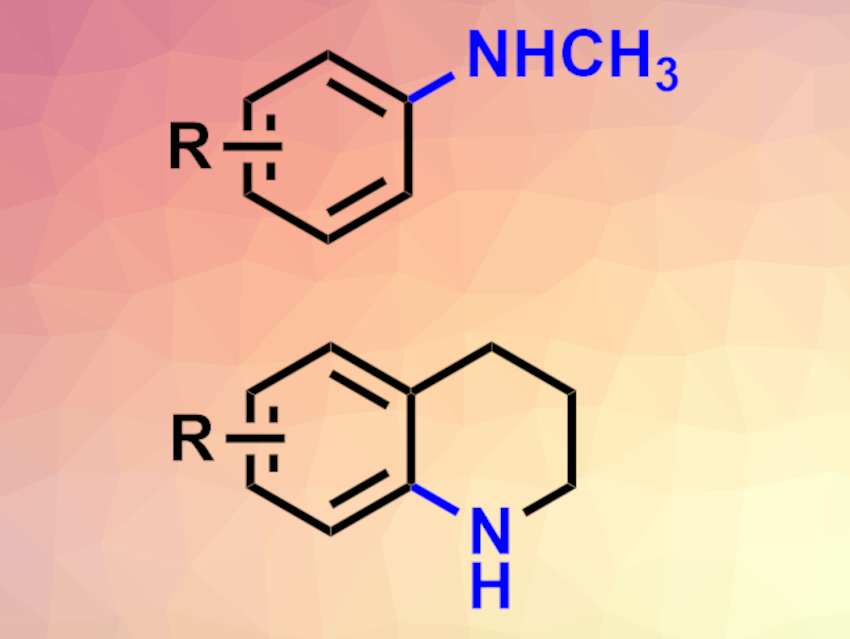
Aromatic amines are commonly used, e.g., in pharmaceutical chemistry, materials chemistry, or dyes. Their synthesis usually requires multistep approaches, harsh reaction conditions, or environmentally harmful transition metals.
Swiss Federal Institute of Technology (ETH) Zurich, Switzerland, and colleagues have developed processes for the synthesis of N-methylanilines and tetrahydroquinolines (pictured) from arenes with an environmentally benign iron(II) salt as a catalyst. The team developed a highly electrophilic aminating reagent of the type [NsO-NH2-CH3]+ (Ns = nosyl) for the reaction. They used FeSO4·7H2O as the catalyst and 1,1,1,3,3,3-hexafluoroisopropanol (HFIP) as the solvent. The reaction can be performed under air at 40 °C.
Using this approach, the team converted a variety of electron-neutral and electron-rich arenes to the corresponding N-methylaniline derivatives in moderate to good yields. The reaction can also be used in an intramolecular manner to prepare tetrahydroquinolines. For this, the researchers used arenes with a propyl sidechain, functionalized with an –N(OTs)Boc (TS = tosyl) group at the end, and added trifluoroacetic acid (TFA). The desired tetrahydroquinoline derivatives were obtained in moderate yields.
- Synthesis of N-Alkyl Anilines from Arenes via Iron-Promoted Aromatic C–H Amination,
Eric Falk, Valentina C. M. Gasser, Bill Morandi,
Org. Lett. 2021.
https://doi.org/10.1021/acs.orglett.1c00099