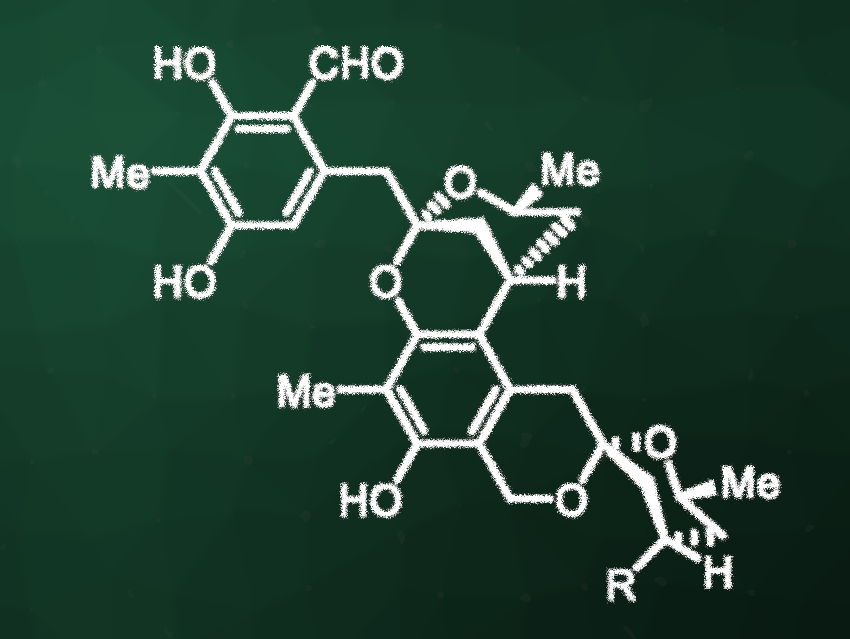
Peniciketals A and B (pictured, R = OH for peniciketal A and R = H for peniciketal B) are structurally complex spiroketals that were originally isolated from the fungus Penicillium raistrickii. They have shown cytotoxic activity against, e.g., human leukemia and lung cancer cell lines. The compounds possess a benzannulated [6,6]-spiroketal unit and a benzo-fused 2,8-dioxabicyclo[3.3.1]nonane moiety. Due to their complex structures, they are challenging to synthesize.
Amos B. Smith III, University of Pennsylvania, Philadelphia, USA, and colleagues have performed the first enantioselective total syntheses of (+)-peniciketals A and B. The team used Type I Anion Relay Chemistry (ARC) to construct the benzannulated [6,6]-spiroketal skeleton. ARC is a useful approach for creating complex chemical scaffolds in one step and a stereocontrolled manner via the migration of negative charge. A reaction sequence that incorporates a Negishi cross-coupling and an olefin cross-metathesis reaction was used to create the trans-enone structure. The researchers then used a photoisomerization/cyclization tactic to couple the resulting fragments and obtain the desired overall structure.
The total synthesis of (+)-peniciketal A has a longest linear sequence of 17 steps. (+)-Peniciketal B was obtained in eight steps from a spiroketal intermediate in the (+)-peniciketal A synthesis, with an overall yield of 24 %. The spectral properties of the products were identical to the respective natural peniciketals.
- Enantioselective Total Synthesis of (+)-Peniciketals A and B: Two Architecturally Complex Spiroketals,
Yifan Deng, Chia-Ping H. Yang, Amos B. Smith III,
J. Am. Chem. Soc. 2021.
https://doi.org/10.1021/jacs.0c11424