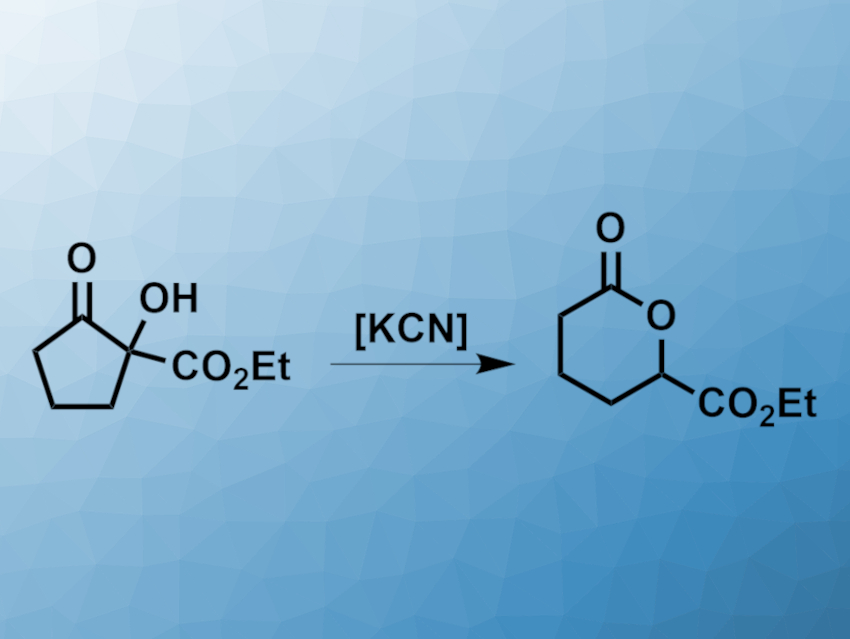
Lactones, i.e., cyclic carboxylic esters, are found in many natural products and biologically active compounds. δ-Lactones, for example, are common structural motifs. They can be synthesized, e.g., by the intramolecular esterification of δ-hydroxy acids or via a Baeyer–Villiger oxidation.
David Kieslich and Jens Christoffers, University of Oldenburg, Germany, have developed an approach for the transformation of α-hydroxy-β-oxoesters to δ-lactones with an additional ester group in the δ-position (pictured). The team originally attempted an etherification of an α-hydroxy-β-oxoester in the presence of a base and ammonium iodide. This reaction was unsuccessful, but the corresponding δ-lactone was isolated from the reaction mixture with a yield of 17 %. The researchers then optimized the reaction conditions and found that with KCN as a catalyst and boiling toluene as the solvent, δ-lactone yields up to 99 % can be achieved.
The α-hydroxy-β-oxoesters can be prepared by a cerium-catalyzed aerobic oxidation of β-oxoesters. This easy accessibility of the starting materials is an advantage of the developed δ-lactone synthesis. According to the researchers, the reaction proceeds via a retro-Dieckmann reaction that opens the five-ring, followed by a lactonization to give the desired products.
- Formation of δ-Lactones by Cyanide Catalyzed Rearrangement of α-Hydroxy-β-oxoesters,
David Kieslich, Jens Christoffers,
Org. Lett. 2021.
https://doi.org/10.1021/acs.orglett.0c04157