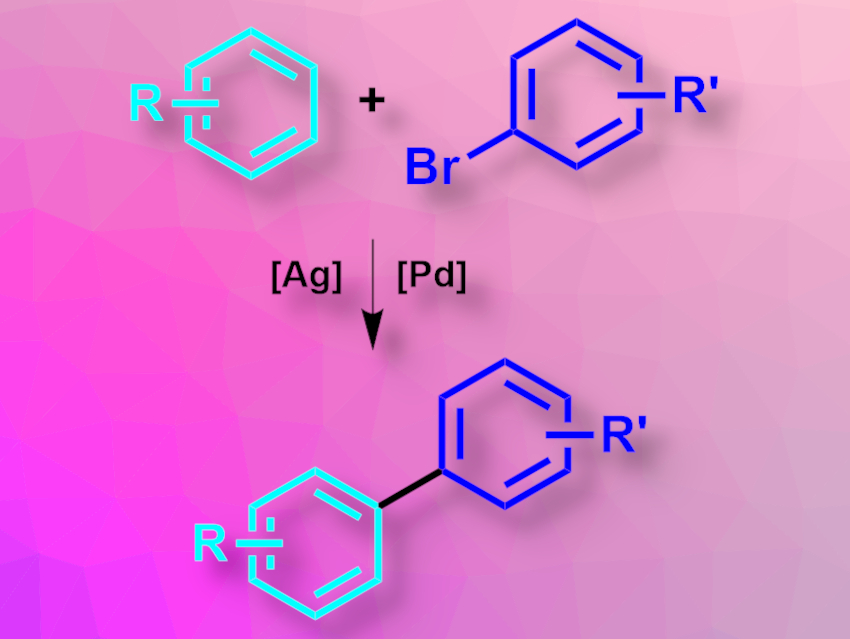
Biaryl units are commonly found, e.g., in pharmaceutically active compounds or agrochemicals. They can, for example, be synthesized via the direct arylation of arenes with aryl electrophiles. However, the direct intermolecular arylation of simple arenes without directing groups is challenging.
John F. Hartwig, University of California, Berkeley, USA, and colleagues have developed a method for the direct arylation of simple arenes with aryl bromides that does not require directing groups (pictured). The reaction uses a catalyst system based on silver and palladium, which act synergistically. The team reacted a variety of substituted arenes with different aryl bromides (with a small excess of the arene) in the presence of Pd(OAc)2 as a catalyst, Ag2O as both catalyst and base, tBuPCy2 (Cy = cyclohexyl) as a ligand, Cs2CO3 as a base, and tert-amyl alcohol as the solvent. The reaction was performed at 120 °C.
The desired biaryl products were obtained in moderate to good yields. According to the researchers, the mechanism likely involves first cleaving the aryl C–H bond with phosphine-ligated silver complexes, followed by the palladium-catalyzed formation of the biaryl compounds. The work could be a basis for the further development of undirected C–H bond functionalization reactions.
- Direct Arylation of Simple Arenes with Aryl Bromides by Synergistic Silver and Palladium Catalysis,
Adrian Tlahuext-Aca, Sarah Yunmi Lee, Shu Sakamoto, John F. Hartwig,
ACS Catal. 2021.
https://doi.org/10.1021/acscatal.0c05254