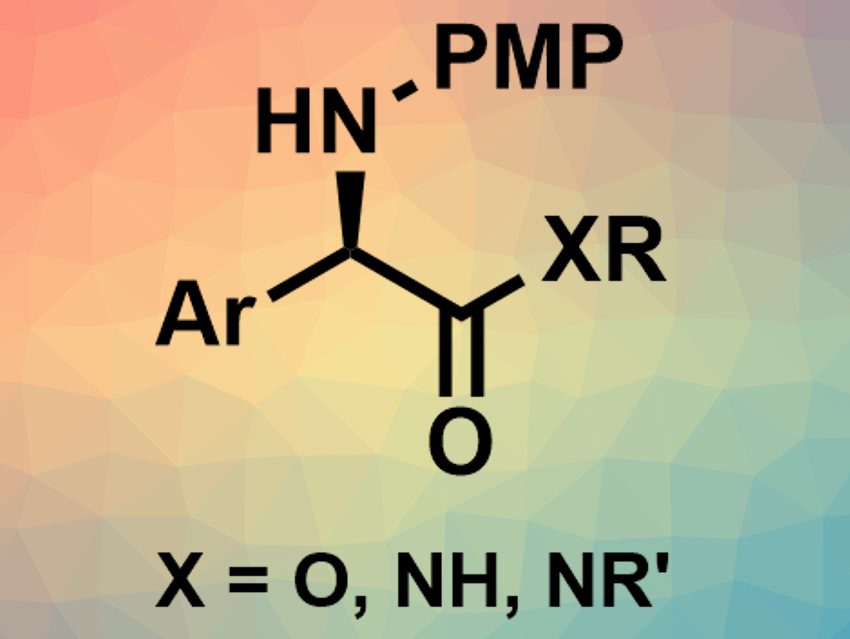
Chiral α-amino acids have applications, e.g., in pharmaceutical chemistry. Chiral α-aryl glycines, for example, are found in both bioactive natural products and synthetic drugs. They can be prepared, e.g., via a transition-metal-catalyzed asymmetric hydrogenation of α-aryl imino esters. However, existing methods for this type of reaction have drawbacks such as a limited substrate scope. Substrates with an easy-to-remove N–p-methoxyphenyl (PMP) protection group can help to overcome this limitation.
Wanbin Zhang, Shanghai Jiao Tong University, China, and colleagues have developed an efficient asymmetric hydrogenation of N-PMP imino esters for the synthesis of useful chiral α-aryl glycines (pictured), catalyzed by earth-abundant nickel. The team used Ni(OAc)2·4H2O as a catalyst, together with chiral ligands of the type (R,R)-BenzP* (BenzP* = 1,2-bis(t-butylmethylphosphino)benzene), to convert a variety of N-aryl imino esters into the corresponding chiral α-aryl glycines under 30 bar of H2. Using amides instead of esters as substrates was also possible.
The desired products were obtained in high yields and with excellent enantioselectivities, and the reaction could be used on a gram scale. The chiral products can be easily deprotected and used for further transformations.
- Ni-catalyzed asymmetric hydrogenation of N-aryl imino esters for the efficient synthesis of chiral α-aryl glycines,
Dan Liu, Bowen Li, Jianzhong Chen, Ilya D. Gridnev, Deyue Yan, Wanbin Zhang,
Nat. Commun. 2020.
https://doi.org/10.1038/s41467-020-19807-5