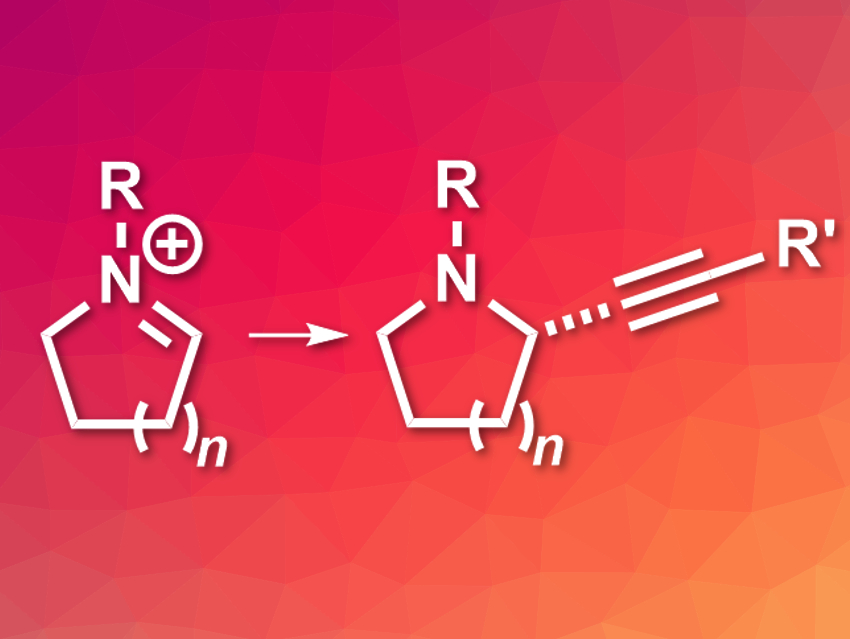
Nitrogen-containing heterocycles are important building blocks in organic synthesis and drug discovery. The stereoselective synthesis of substituted cyclic amines, for example, can lead to useful bioactive compounds. This can be achieved by enantioselective alkynylations of prochiral cyclic iminium ions. However, the scope of this type of reaction had been limited to resonance-stabilized iminium ions so far, e.g., isoquinolinium, quinolinium, and pyridinium ions.
Mary P. Watson, University of Delaware, USA, and colleagues have developed an enantioselective copper-catalyzed alkynylation of unstabilized cyclic iminium ions (pictured). The team generated the iminium ions in situ from readily available hemiaminal methyl ethers. They used CuI as a catalyst, together with a PyBOX-type chiral bis(oxazoline) ligand, 1,2,2,6,6-pentamethylpiperidine (PMP) as a base, BF3·OEt2 as a Lewis acid, and dimethoxyethane (DME) as the solvent to react these iminium ions with terminal alkynes. The reactions were performed at –50 °C.
The desired alkynylated products were obtained in excellent yields and with good enantioselectivities. The method has a good functional group tolerance and is suitable for the preparation of five- and six-membered nitrogen heterocycles with α-stereocenters.
- Enantioselective Alkynylation of Unstabilized Cyclic Iminium Ions,
Weiye Guan, Samantha O. Santana, Jennie Liao, Kelci Henninger, Mary P. Watson,
ACS Catal. 2020.
https://doi.org/10.1021/acscatal.0c04223