Beryllium is a very light metal with high electronegativity, and Be2+ is the smallest metal ion. This makes the chemistry of beryllium unique and interesting. However, this chemistry has not been well-studied due to the element’s high toxicity. The halides BeCl2, BeBr2, and BeI2 are commonly used starting materials in the study of beryllium chemistry, but even these compounds’ structures and properties have not been extensively investigated.
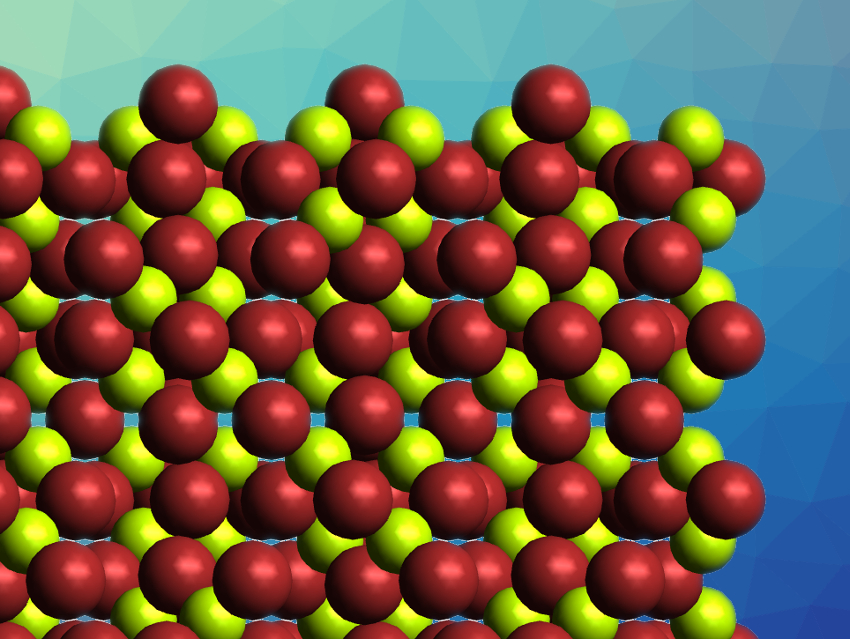
For BeBr2, only a single phase is obtained when the compound is synthesized from these elements. This modification, α-BeBr2, contains chains of tetrahedrally coordinated Be atoms, μ2-linked by two Br atoms each. This α-BeBr2 phase is isostructural to α-BeCl2. BeCl2 has a second modification, β-BeCl2, in which the Be atoms are also tetrahedrally coordinated by the halogen atoms, but these tetrahedra are corner-connected to form supertetrahedra in a 3D network. Analogous α- and β-modifications are known for BeI2. However, a BeBr2 phase that is isostructural to β-BeCl2 and β-BeI2 had been unknown so far.
Magnus R. Buchner, Carsten von Hänisch, University of Marburg, Germany, and colleagues have synthesized β-BeBr2 (pictured) via the recrystallization of α-BeBr2 from benzene in the presence of cyclo-decamethylpentasiloxane as a solubilizer. The product was characterized using single-crystal X-ray diffraction and IR and Raman spectroscopy. The team found that β-BeBr2 is isostructural to β-BeCl2 and β-BeI2, with supertetrahedra forming a three-dimensional coordination network.
- A Second Modification of Beryllium Bromide: β-BeBr2,
Magnus R. Buchner, Fabian Dankert, Nils Spang, Florian Pielnhofer, Carsten von Hänisch,
Inorg. Chem. 2020.
https://doi.org/10.1021/acs.inorgchem.0c02832