Terminal metal nitride complexes are interesting synthesis targets and could be useful, e.g., in the study of catalytic processes involving nitride materials. Complexes with terminal uranium nitride (–U≡N) bonds, for example, are interesting in this context but have proven challenging to synthesize. There is only one example of terminal U(V) or U(VI) nitrides so far [1].
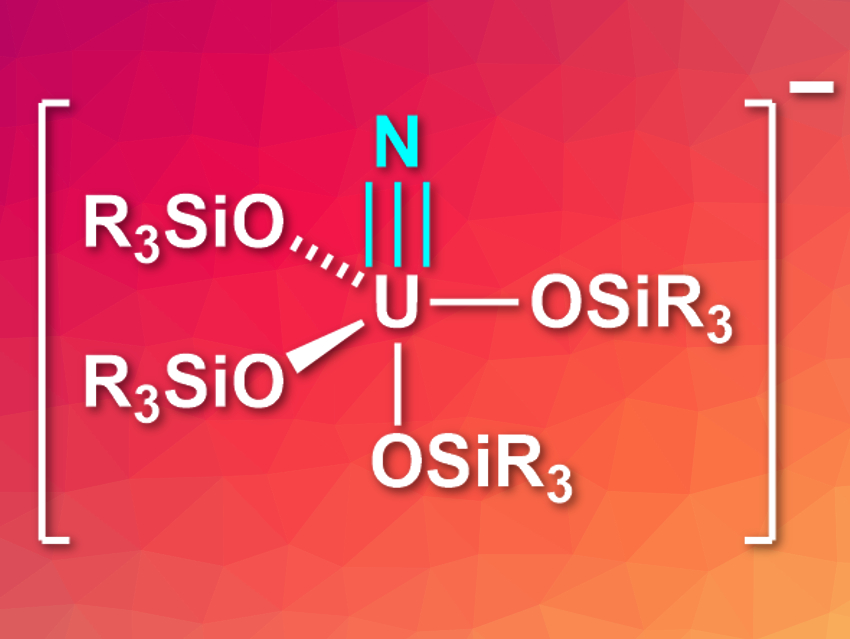
Marinella Mazzanti, Ecole Polytechnique Fédérale de Lausanne (EPFL), Switzerland, and colleagues have synthesized the second example of a terminal U(VI) nitride, [U(OSi(OtBu)3)4(N)]– (pictured). The team first synthesized the sterically demanding terminal U(IV) azide [NBu4][U(OSi(OtBu)3)4(N3)] by reacting the U(IV) tetrasiloxide complex [U(OSi(OtBu)3)4] with the azide NBu4N3. This azide intermediate was then subjected to photolysis in a tetrahydrofuran (THF) solution using UV light. The researchers obtained single crystals of the desired terminal nitride complex salt [NBu4][U(OSi(OtBu)3)4(N)] in 70 % yield.
The nitride complex is stable in solution for weeks, but decomposes under longer UV exposure. It is the first example of an isolated terminal uranium nitride complex prepared via a photochemical reaction. X-ray crystallography shows that the uranium atom is coordinated in a slightly distorted trigonal bipyramidal structure, with the nitride ligand in an axial position. The complex can react with proton sources to give ammonia. It can also react with CO, giving the complex [NBu4][U(OSi(OtBu)3)4(NCO)].
- Photochemical Synthesis of a Stable Terminal Uranium(VI) Nitride,
Luciano Barluzzi, Rosario Scopelliti, Marinella Mazzanti,
J. Am. Chem. Soc. 2020.
https://doi.org/10.1021/jacs.0c09814
Reference
- Synthesis and Structure of a Terminal Uranium Nitride Complex,
D. M. King, F. Tuna, E. J. L. McInnes, J. McMaster, W. Lewis, A. J. Blake, S. T. Liddle,
Science 2012, 337, 717–720.
https://doi.org/10.1126/science.1223488