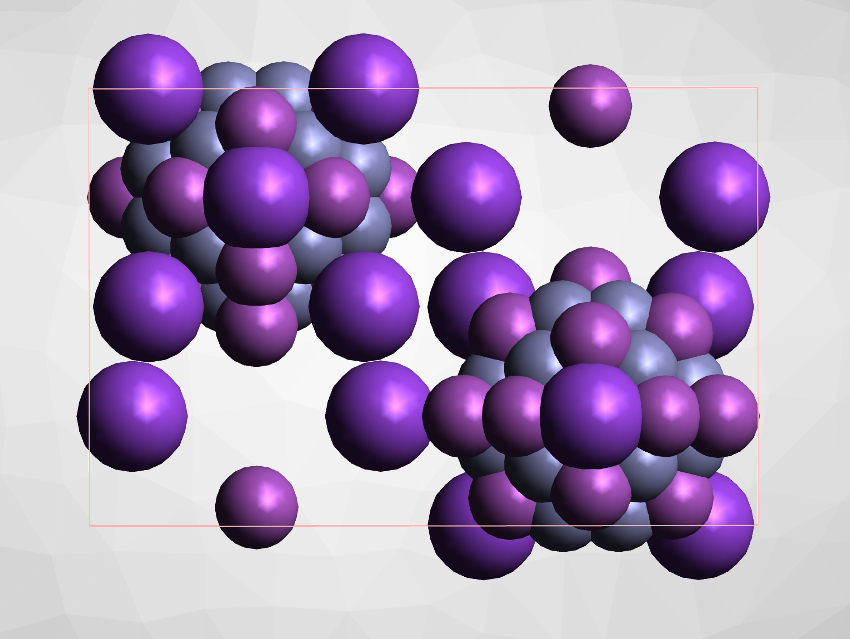
Zinc is usually found in its stable +II oxidation state. Low-valent zinc compounds and clusters are rare, but can have interesting properties and reactivities. Metals such as zinc do not form metal–metal bonds and low-valent-metalloid clusters easily.
Florian Weigend, University of Marburg, Germany, and Karlsruhe Institute of Technology (KIT), Eggenstein-Leopoldshafen, Germany, Stefanie Dehnen, University of Marburg, and colleagues have synthesized the heterometallic cluster anion [K2Zn20Bi16]6–, which contains a rare metalloid Zn12 unit at its core. The team reacted K5Ga2Bi4 with ZnPh2 in ethane-1,2-diamine in the presence of [2.2.2]-cryptand at room temperature and obtained [K(crypt-222)]6[K2Zn20Bi16] as a black, crystalline product.
The structure of the cluster anion was studied using X-ray crystallography. It contains a cluster of twelve zinc atoms in its center, surrounded by a cycle consisting of eight Zn atoms and 16 Bi atoms. Quantum-chemical calculations show that the Zn12 cluster has a metalloid character, with four-center bonding connecting the Zn atoms. According to the researchers, the cluster could potentially be useful in catalysis.
- Stabilizing a metalloid {Zn12} unit within a polymetallide environment in [K2Zn20Bi16]6−,
Armin R. Eulenstein, Yannick J. Franzke, Patrick Bügel, Werner Massa, Florian Weigend, Stefanie Dehnen,
Nat. Commun. 2020.
https://doi.org/10.1038/s41467-020-18799-6