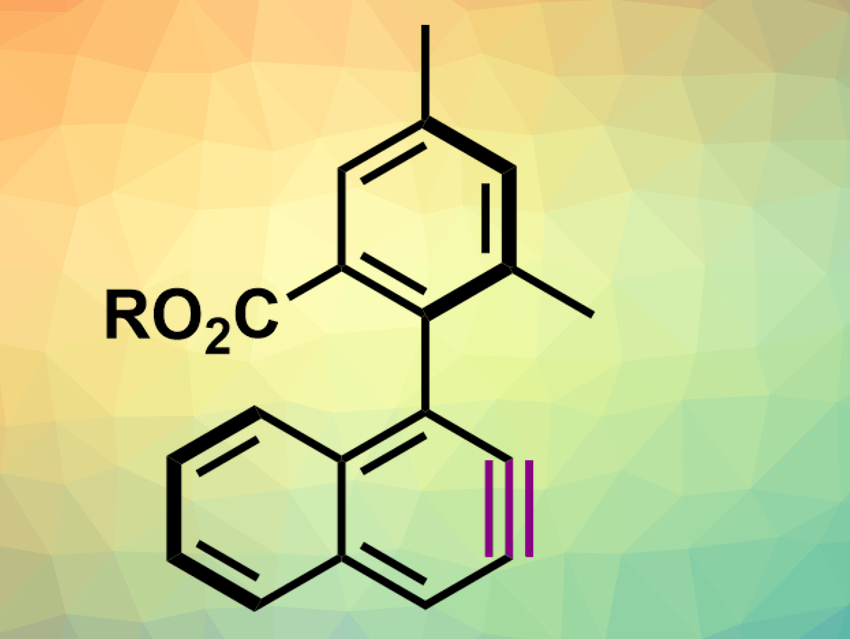
Ortho-Arynes or benzynes are reactive intermediates and can be described as benzene derivatives with a strained triple bond. They can easily undergo reactions such as nucleophilic additions or cycloadditions. Due to their high reactivity, arynes need to be generated in situ from suitable aromatic precursors. Usually, these precursors and the resulting arynes are achiral.
Jean Rodriguez, Yoann Coquerel, and colleagues, Aix-Marseille University, Marseille, France, have created enantioenriched aryne atropisomers with a biaryl-type chiral axis vicinal to the aryne triple bond (example pictured). This type of aryne can be synthesized from an enantioenriched precursor with neighboring iodo- and triflate substituents via a reaction with trimethylsilylmethyl magnesium chloride in diethyl ether (Et2O). The reactive, enantioenriched amine can then be trapped using, e.g., furan in a Diels–Alder cycloaddition reaction.
The team found that the enantiomeric purity of the axially chiral aryne precursor (ca. 90 %) was retained after the conversion to an aryne and the subsequent trapping reaction. This approach provides new options for the stereocontrolled synthesis of atropisomers.
- Enantiospecific Generation and Trapping Reactions of Aryne Atropisomers,
Yun-Long Wei, Guillaume Dauvergne, Jean Rodriguez, Yoann Coquerel,
J. Am. Chem. Soc. 2020.
https://doi.org/10.1021/jacs.0c08218