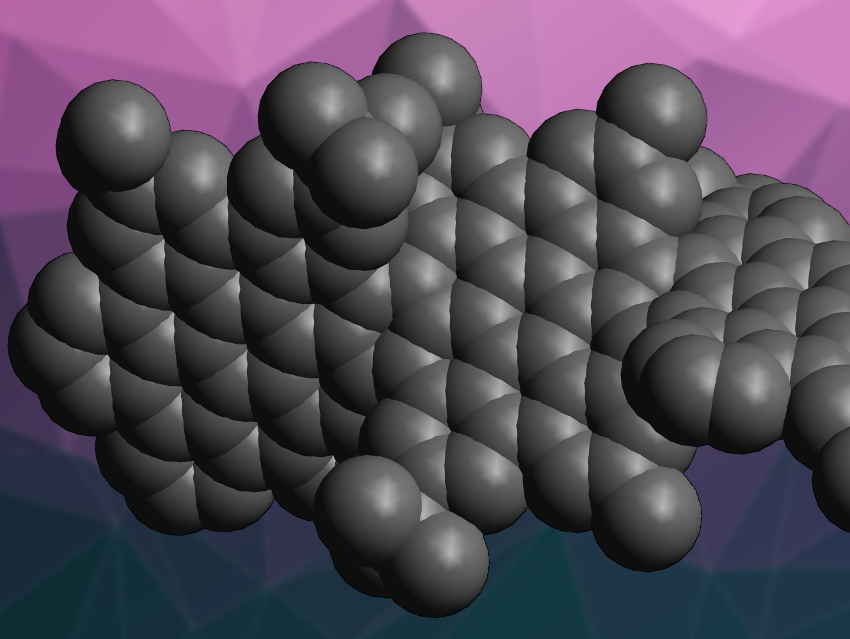
Twistacenes are polycyclic aromatic hydrocarbons with a helical shape. Due to this shape, they are chiral. However, twistacenes are prone to racemization, which prevents investigations of their chiroptical properties. Replacing the fused benzene rings of twistacenes with larger units could result in systems with stable configurations.
Jiaobing Wang, Sun Yat-Sen University, Guangzhou, China, and colleagues have used superbenzene units (hexabenzocoronenes, HBCs) to synthesize a helical graphene nanoribbon, which the team calls “supertwistacene”. It was synthesized via a polyphenylene intermediate, which was oxidized to form the central polycyclic system and extended at both ends using Sonogashira coupling reactions. Diels–Alder reactions at both ends, followed by a dehydrocyclization, then gave the desired product.
The nanoribbon consists of four linearly fused HBC units, has an overall twist of 117°, and a length of 4.3 nm. Bulky tert-butyl substitutions at the ribbon’s edges prevent racemization. Single enantiomers of the nanoribbon were obtained using chiral high-performance liquid chromatography (HPLC). The optically pure compound showed a strong Cotton effect, i.e., a change in circular dichroism around its absorption bands. According to the team, this work could be a basis for further studies of nonplanar polycyclic aromatic hydrocarbons.
- Supertwistacene: A Helical Graphene Nanoribbon,
Shuang Ma, Jiajian Gu, Chaojun Lin, Zhixing Luo, Yanpeng Zhu, Jiaobing Wang,
J. Am. Chem. Soc. 2020.
https://doi.org/10.1021/jacs.0c08555