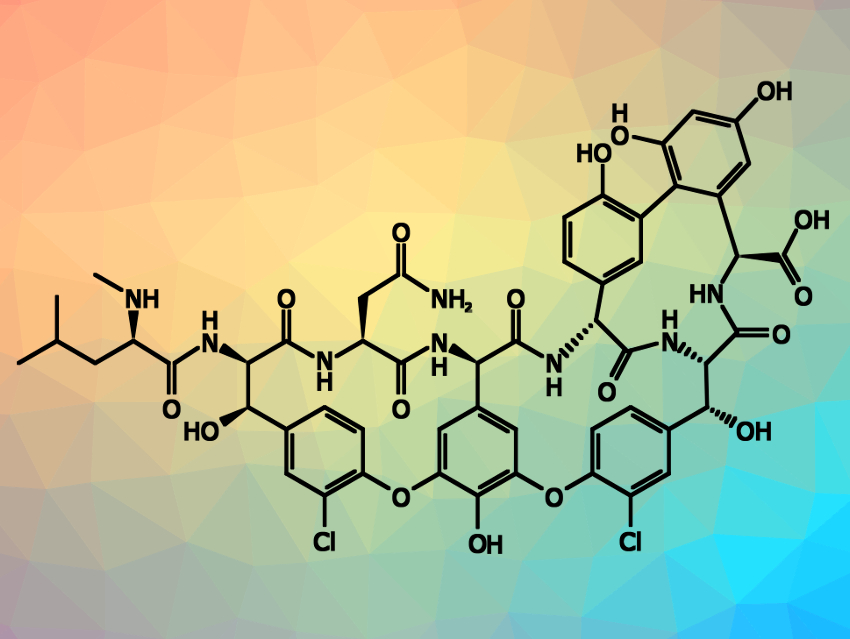
Vancomycin is an important antibiotic for the treatment of bacterial infections—in particular, for potentially life-threatening infections with bacteria that are resistant to other drugs. It consists of three macrocyclic rings attached to a peptide core, decorated with glycosyl groups. The derivative without these glycosyl groups is called vancomycin aglycon (pictured). Vancomycin has been prepared before, but the existing synthesis paths require many steps and provide low yields. An improved synthesis of vancomycin aglycon and its derivatives could allow researchers to study the effectiveness of new vancomycin analogues as antibiotics.
Dale L. Boger, The Scripps Research Institute, La Jolla, CA, USA, and colleagues have developed an improved total synthesis of vancomycin aglycon that requires only 17 steps (longest linear sequence). The team used, e.g., a one-pot Miyaura borylation–Suzuki coupling sequence to introduce the axially chiral biaryl unit in a stereoselective manner, a scalable macrolactamization to close one of the ring systems, and two intramolecular SNAr cyclizations to close the other two macrocycles. Another two steps are then required for the enzymatic glycosylation of vancomycin aglycon, leading to a total of 19 steps for the synthesis of vancomycin.
The team’s approach reduces the number of steps from the previous best of 27 (longest linear sequence) and increases the overall yield to 3.7 %, compared with up to 0.14 % for previous methods. It could allow researchers to study vancomycin analogues and search for drug candidates that are effective even against vancomycin-resistant bacteria.
- Next-Generation Total Synthesis of Vancomycin,
Maxwell J. Moore, Shiwei Qu, Ceheng Tan, Yu Cai, Yuzo Mogi, D. Jamin Keith, Dale L. Boger,
J. Am. Chem. Soc. 2020.
https://doi.org/10.1021/jacs.0c07433