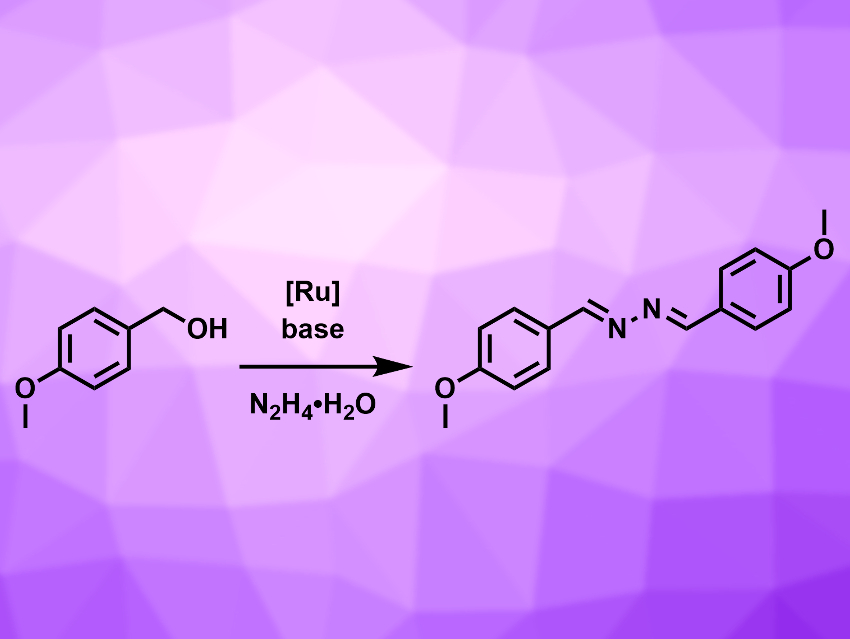
Azines are molecules containing a –C=N–N=C– unit. They are useful building blocks for metal–organic frameworks and supramolecular structures and can serve as intermediates in the synthesis of medically important molecules. Some azines have biological activities and are candidates for drug development, e.g., as agents against hypertension or fungal infections. Known synthesis methods for azines often have drawbacks such as a limited substrate scope, low atom economy, or the need for inert reaction conditions.
Rengan Ramesh, Bharathidasan University, Tiruchirappalli, India, and colleagues have developed a direct synthesis route for symmetrical azines from alcohols and hydrazine hydrate (N2H4•H2O), catalyzed by Ru(II) complexes with arene and aroylthiourea ligands. The aroylthiourea ligands are easily accessible, air-stable, and form a stable N,S-chelate structure with the Ru center. The team used aryl alcohols as substrates, [RuCl(HL)(η6–p-cymene)]Cl (HL = N-(pyrimidin-2-ylcarbamothioyl)furan-2-carboxamide) as the catalyst, t-BuOK as a base, and toluene as the solvent. The reaction was carried out at 80 °C under air using molecular sieves to remove water from the reaction mixture. The addition of a base leads to higher yields, but is not essential for the reaction.
The reaction has a large substrate scope and gives the azine products in good yields. It is highly atom-economic, with the only byproduct being water. The process can be performed under air, which avoids the need for inert conditions, and the reaction is completed in one step. Overall, this method is a useful approach for the synthesis of azines from aryl-substituted alcohols.
- Non-Pincer-Type Arene Ru(II) Catalysts for the Direct Synthesis of Azines from Alcohols and Hydrazine under Aerobic Conditions,
Sundar Saranya, Rengan Ramesh, David Sémeril,
Organometallics 2020.
https://doi.org/10.1021/acs.organomet.0c00367
Correction (August 27, 2020)
The image originally showed a phenol instead of a benzyl alcohol; this has been corrected.