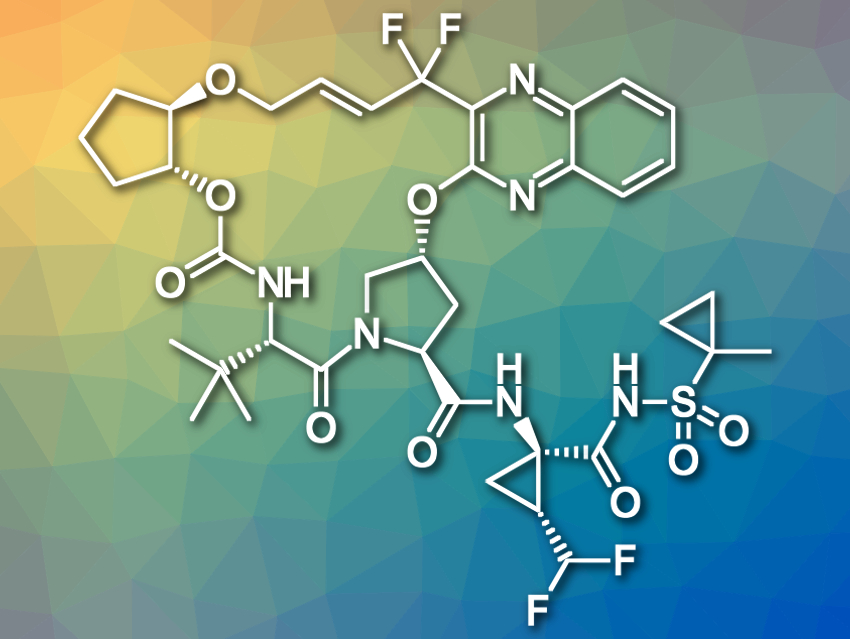
Glecaprevir (pictured) is a hepatitis C virus protease inhibitor which has successfully undergone clinical trials. However, the synthesis route that was used to make glecaprevir for early drug development is not suitable for large-scale production, which is necessary for late-stage clinical trials and broad application in the treatment of persistent cases of hepatitis C. Thus, a different, scaleable synthesis was needed.
Russel D. Cink, AbbVie Inc., North Chicago, IL, USA, and colleagues have developed an improved synthesis route for the macrocyclic unit of glecaprevir (pictured top left). One synthetically challenging part of this molecule is the gem-difluoromethylene group. Forming this structure from an advanced intermediate is problematic due to the costs and difficulties of handling suitable fluorinating agents on a large scale. To avoid this, the team chose commercially available ethyl trifluoropyruvate as an already fluorinated starting material.
The ring-closing of the macrocycle was achieved using an ether formation between an allylic bromide and a hydroxy-substituted carbamate building block. The allylic bromide was formed during the final stages of the synthesis by conversion of on allylic alcohol using PPH3Br2 as a brominating agent. This was followed by the cyclization, which was catalyzed by the base Triton B (BnMe3NOH).
In pharmaceutical chemistry, effective purification of the products is particularly important, because impurities can lead to negative health effects. The first attempts to purify the macrocyclic product took an entire week per batch. Due to the impracticality of this process, the team developed a crystallization process using a mixture of p-xylene and water. The overall yield of the developed synthesis route is 20 % (improved from 15 % in the previously used route), and the scale limitations of the original synthesis could be overcome. Starting from the synthesized macrocycle, the team completed the synthesis of glecaprevir on a scale that allowed late-stage clinical development [1].
- Development of a Large-Scale Route to Glecaprevir: Synthesis of the Macrocycle via Intramolecular Etherification,
Jeffrey M. Kallemeyn, Kenneth M. Engstrom, Matthew J. Pelc, Kirill A. Lukin, Westin H. Morrill, Haojuan Wei, Timothy B. Towne, Jeremy Henle, Nandkishor K. Nere, Dennie S. Welch, Shashank Shekhar, Matthew M. Ravn, Gang Zhao, Michael G. Fickes, Chen Ding, John C. Vinci, James Marren, Russell D. Cink,
Org. Process Res. Dev. 2020.
https://doi.org/10.1021/acs.oprd.0c00244
Reference
- [1] Development of a Large-Scale Route to Glecaprevir: Synthesis of the Side Chain and Final Assembly,
David R. Hill, Michael J. Abrahamson, Kirill A. Lukin, Timothy B. Towne, Kenneth M. Engstrom, Rajarathnam E. Reddy, Angelica B. Kielbus, Matthew J. Pelc, Jianzhang Mei, Nandkishor K. Nere, Shuang Chen, Rodger Henry, Sanjay Chemburkar, Chen Ding, Hongqiang Zhang, Russell D. Cink,
Org. Process Res. Dev. 2020.
https://doi.org/10.1021/acs.oprd.0c00245