Nanosized macromolecules that can act as hosts in host–guest complexes can have applications, e.g., in sensing. They are often made from aromatic systems, which introduces strain when these systems are bent to form, e.g., nanorings or nanotubes. Nanorings such as cyclo-para-phenylenes (CPPs), for example, have been synthesized from non-aromatic precursors to avoid this strain for most of the synthesis. However, extending this type of compound to form nanotubular structures can be challenging.
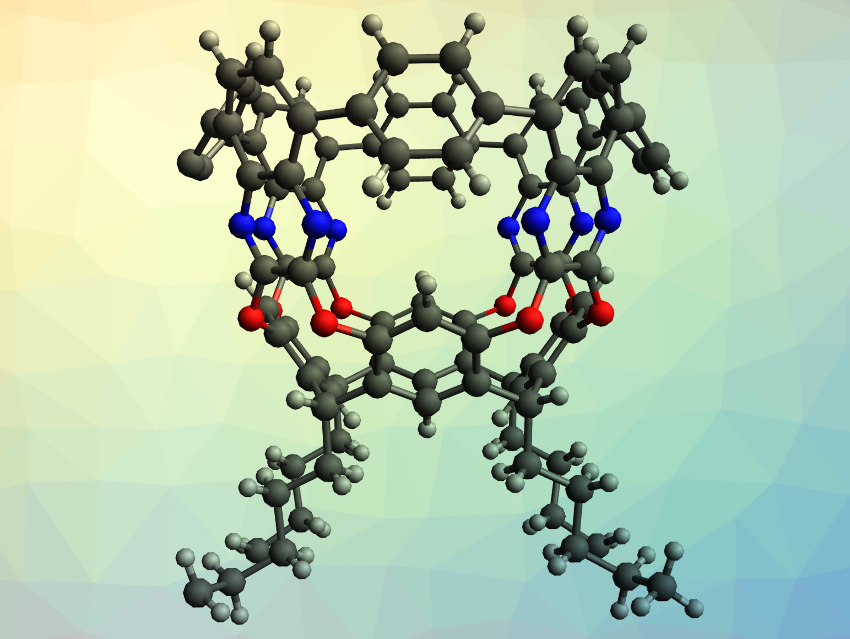
Raúl Hernández Sánchez and colleagues, University of Pittsburgh, PA, USA, have synthesized highly-strained aromatic compounds with a tubular shape, which the team calls tubularenes (example pictured). The team started their synthesis from resorcinarenes, macrocycles that are made from aldehydes and resorcins. They synthesized tubular[4,8,8,8]arene (pictured) and tubular[4,8,8,12]arene, which differ only in the number of aromatic rings connected to the resorcinarene base. The resorcinarene was first reacted with 2,3-dichloro-5,8-dibromoquinoxaline to extend the “height” of the structure. Then, Suzuki–Miyaura cross-couplings with either 1,4-benzenediboronic- or 1,4-naphthalenediboronic acid bis(pinacol) ester led to a ring closing that forms the tubular structure. The reaction with the benzene-based diester gives tubular[4,8,8,8]arene, while the reaction with the larger naphthalene-based diester gives tubular[4,8,8,12]arene.
The synthesized tubularenes have rigid frameworks with permanent cavities. The compounds are also electrochemically active and can accept up to four electrons. Due to the modular synthesis, the structure and properties of this class of compounds should be easily tunable for different applications.
- Tubularenes,
Saber Mirzaei, Edison Castro, Raúl Hernández Sánchez,
Chem. Sci. 2020.
https://doi.org/10.1039/d0sc03384g


![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)
