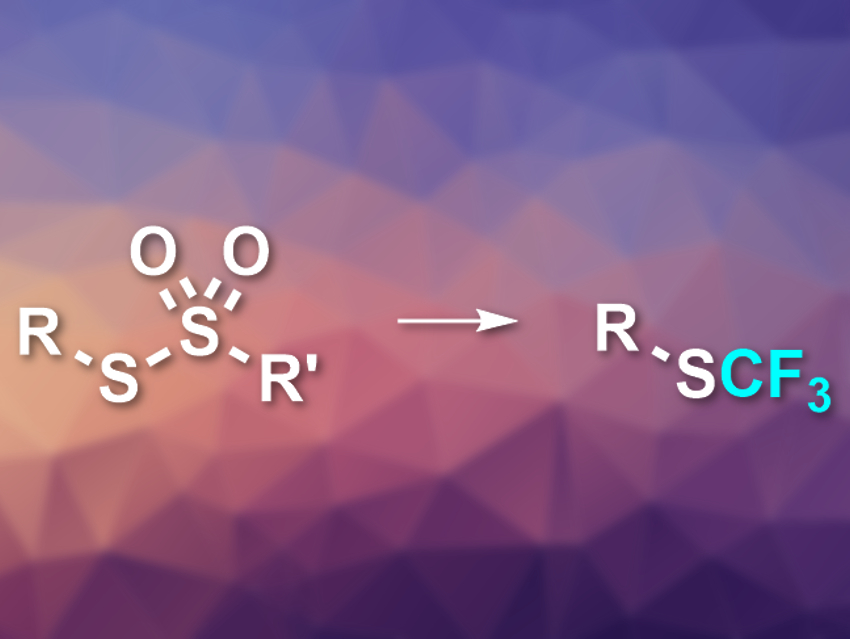
Trifluoromethylthio (−SCF3) and related groups are often useful to improve the properties of drug candidates. Methods to introduce these groups to organic compounds can involve hazardous, expensive, or hard-to-make reagents. A better approach might be the use of nucleophilic trifluoromethylating reagents to convert sulfur electrophiles into the desired RSCF3-type compounds. However, there is a lack of suitable electrophiles for this reaction.
Gavin Chit Tsui, The Chinese University of Hong Kong, Shatin, Hong Kong SAR, and colleagues have used thiosulfonates as sulfur electrophiles for nucleophilic perfluoroalkylation reactions (pictured). The team used a range of easy-to-access thiosulfonates and reacted them with an excess of the commercially available trifluoromethyltrimethylsilane (TMS–CF3, also known as the Ruppert-Prakash reagent), together with NaOAc as an initiator. The reaction was performed in dimethyl sulfoxide (DMSO) at room temperature.
The desired RSCF3-type compounds were obtained in moderate to excellent yields. Both aryl- and alkyl-substituted perfluoroalkyl sulfides can be prepared. The protocol is transition-metal-free and does not produce toxic or foul-smelling byproducts, which can occur with other sulfur electrophiles. The reaction can also be used to introduce longer perfluoroalkyl groups such as –CF2CF3 or –(CF2)2CF3 when the corresponding reagents of the type TMS(CF2)nCF3 are used.
- Perfluoroalkylation of Thiosulfonates: Synthesis of Perfluoroalkyl Sulfides,
Ziwei Luo, Xinkan Yang, Gavin Chit Tsui,
Org. Lett. 2020.
https://doi.org/10.1021/acs.orglett.0c02235