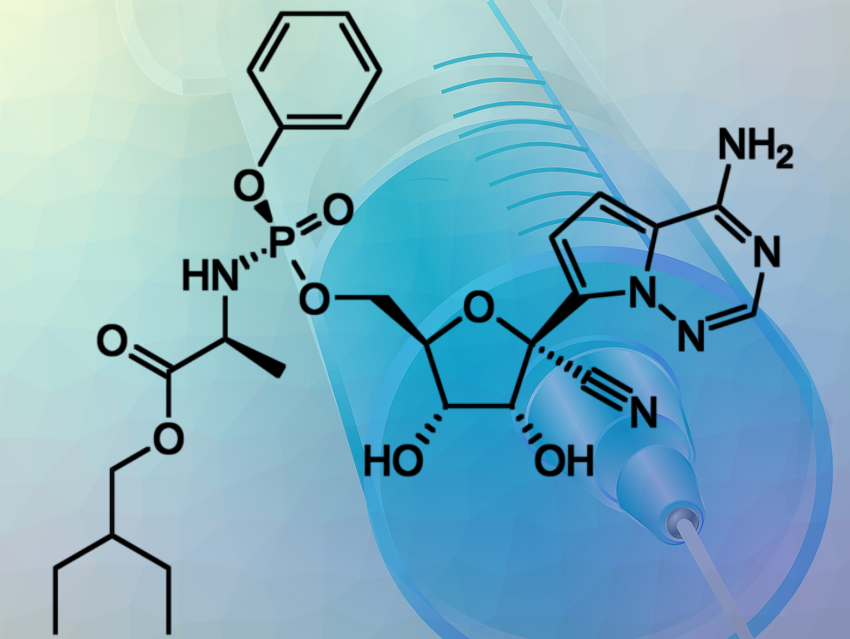
Gilead announced that the European Commission has granted conditional marketing authorization for Veklury® (remdesivir) as a treatment for SARS-CoV-2 infection, the virus that causes COVID-19. Under this authorization, Veklury is indicated for the treatment of COVID-19 in adults and adolescents (aged 12 years and older and weighing at least 40 kg), with pneumonia requiring supplemental oxygen.
A conditional marketing authorization in Europe is initially valid for one year but can be extended or converted into an unconditional marketing authorization after the submission and assessment of additional confirmatory data.
Remdesivir was originally developed for the treatment of Ebola but showed too little effect. At the end of April, an international study involving over 1000 participants showed that patients treated with remdesivir recovered after about 11 days, compared with 15 days for patients given placebo. This effect was not observed in patients with mild to moderate disease. Mortality decreased slightly in the study, but this was not statistically significant.
Ongoing clinical trials continue to evaluate the safety and efficacy of remdesivir, including studies of remdesivir in combination with anti-inflammatory medicines and in special populations including pediatric patients. Research is also being conducted on new, investigational formulations of remdesivir that may enable studies of remdesivir in earlier stages of the disease.
Remdesivir has been approved as a treatment for patients with severe COVID-19 in Japan, Taiwan, India, Singapore, and the United Arab Emirates. In the US, remdesivir (GS-5734™) is authorized for use under an Emergency Use Authorization (EUA).
- European Commission (EC), Brussels, Belgium
- Gilead Sciences, Inc., Foster City, CA, USA
Also of Interest
- Collection: SARS-CoV-2 Virus
What we know about the new coronavirus and COVID-19