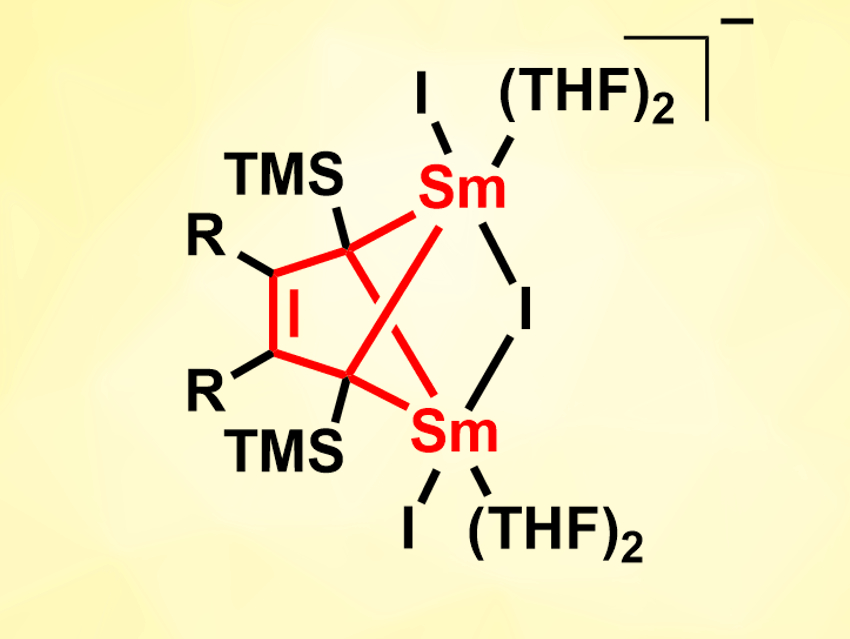
The reduction of alkenes via single-electron transfer (SET) reactions, e.g., with samarium(II) reagents, is useful in organic synthesis. However, this type of reaction is challenging when electron-rich olefins are used. For anionic conjugated olefins, for example, no such SET reduction with divalent rare-earth metal complexes had been reported so far.
Han-Shi Hu, Tsinghua University, Beijing, China, Wen-Xiong Zhang, Peking University, Beijing, China, and colleagues have performed a reduction of anionic conjugated olefins, i.e., 1,3-butadienyl-based dianions, with SmI2(THF)2 (THF = tetrahydrofuran). The 1,3-butadienyl dianions were prepared in situ from the corresponding trimethylsilyl-substituted 1,4-dilithio-1,3-butadienes. The reaction of these precursors with SmI2(THF)2 in THF gives 2-butene-tetraanion-bridged disamarium(III) complexes (pictured, R = Me, Ph, (CH2)4).
Using density functional theory (DFT) calculations, the team found that the tetraanionic 2-butene ligand serves as a ten-electron donor with two σ-type orbitals and three π-type donating orbitals. The complexes show interesting reactivities: The tetraanionic ligand can serve as a three-electron reductant toward cyclooctatetraene (COT), and the complexes can, e.g., undergo salt metathesis with Cp*Li or CO insertion in a reaction with Mo(CO)6.
- 2-Butene Tetraanion Bridged Dinuclear Samarium(III) Complexes via Sm(II)-Mediated Reduction of Electron-Rich Olefins,
Yu Zheng, Chang-Su Cao, Wangyang Ma, Tianyang Chen, Botao Wu, Chao Yu, Zhe Huang, Jianhao Yin, Han-Shi Hu, Jun Li, Wen-Xiong Zhang, Zhenfeng Xi,
J. Am. Chem. Soc. 2020.
https://doi.org/10.1021/jacs.0c01690