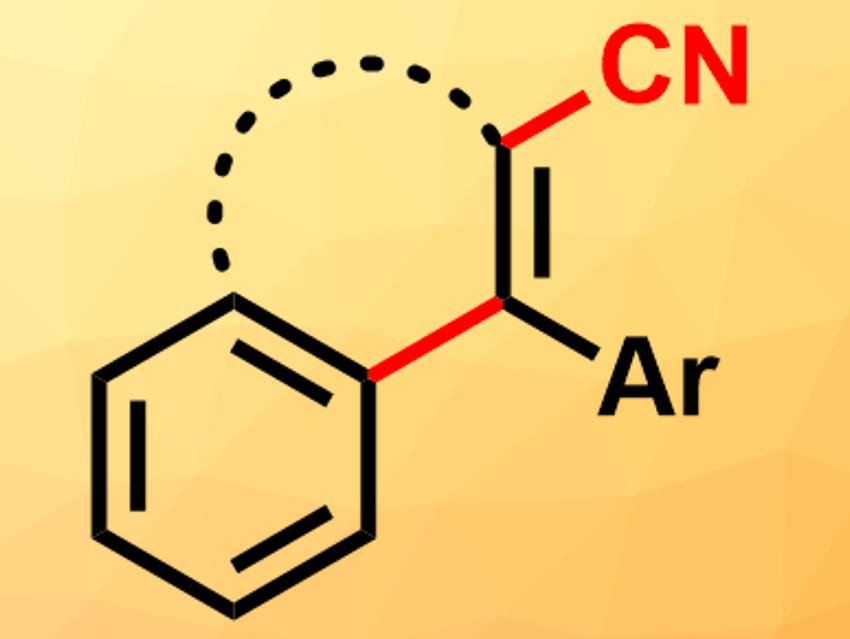
Polycyclic organic systems are part of many compounds found in biological systems. Their synthetic construction in the laboratory, however, can be challenging. One strategy is the use of cascade cyclizations of polyprenoid chains, similar to the reactions performed in biological systems. Usually, these cascades are triggered by protons. The use of other electrophiles instead of protons, such as halonium or sulfenium cations, can allow researchers to introduce additional functional groups during the cyclization. However, carbon-based electrophiles, such as [CN]+ equivalents, have rarely been used for this.
Manuel Alcarazo, University of Göttingen, Germany, and colleagues have developed a cyanative alkenylation reaction that creates polycyclic systems from alkyne-substituted arenes, using imidazolium thiocyanate as a source of [CN]+. The reaction selectively gives the desired cyanated phenanthrene derivatives when BCl3 is added as a Lewis acid and 2,6-di-tert-butylpyridine as a base. Without the addition of a base, 1:1 mixtures of the target product and a byproduct without the cyano group are formed due to a side reaction with protons. The reaction works well with electron-rich, aromatic, or thio-alkyl substituents at the alkyne. It is not successful when alkyl-substituted alkynes are used. However, electron-withdrawing groups at the aryl moiety, such as CF3, are tolerated.
The reaction can also be used for the synthesis of aryl-substituted dihydronaphthalenes and dibenzocyclohepta-1,3,5-trienes. Overall, the method provides access to various cyano-substituted ring systems, which can be further functionalized by a variety of reactions at the cyano group.
- Electrophilic Cyanative Alkenylation of Arenes,
Mingyue Zhao, Alejandro G. Barrado, Kristin Sprenger, Christopher Golz, Ricardo A. Mata, Manuel Alcarazo,
Org. Lett. 2020.
https://doi.org/10.1021/acs.orglett.0c01204