In an ischemic stroke, a blood clot restricts blood flow to parts of the brain, which can cause lasting damage or death. Such a stroke causes the formation of harmful reactive oxygen species (ROS), which damage the nerve cells. One of these ROS is hydrogen peroxide, which can be converted to highly reactive and even more harmful hydroxyl radicals. Catalysts that convert H2O2 to oxygen and water—while avoiding the formation of OH· radicals—could be useful to protect nerve cells from damage during a stroke. Manganese-based complexes have been investigated for this, but they generally lead to the production of unwanted OH· radicals.
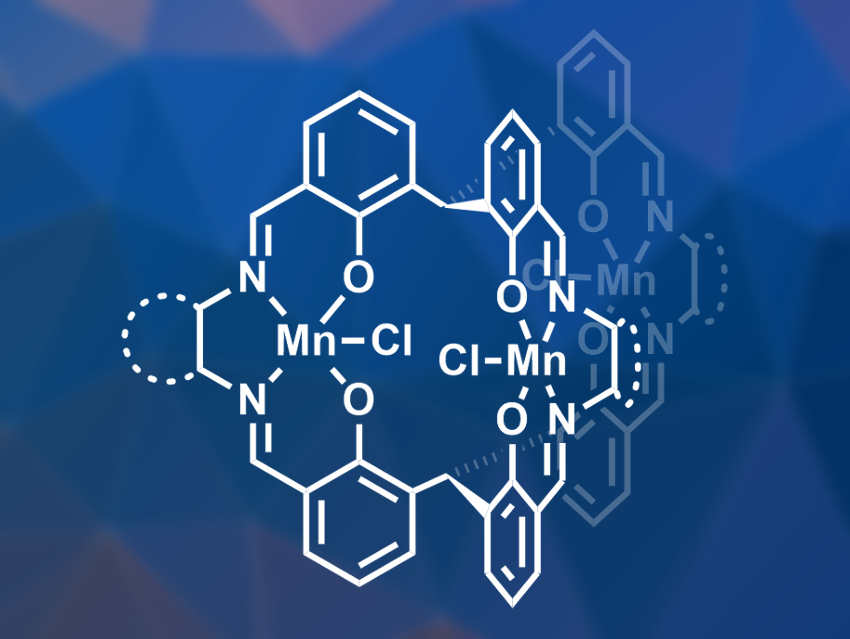
Lei Kang, Jun-Long Zhang, Peking University, Beijing, China, Jonathan L. Sessler, The University of Texas at Austin, USA, and colleagues have developed salen-based tri-nuclear manganese metallocryptands (pictured), which can destroy H2O2 while minimizing the formation of hydroxyl radicals. The complexes were synthesized from a methanetriyl-tris(2-hydroxybenzaldehyde) and one of three different diamine bridges, namely o-phenylenediamine, 1,2-diaminocyclohexane, or ethylenediamine, in the presence of manganese acetate and chloride ions.
The team found that the complexes can promote the conversion of H2O2 to O2 and H2O in an aqueous solution at physiological pH. The o-phenylenediamine-bridged complex showed the most promising results. According to the researchers, the neighboring metal units in the complex have a cooperative effect in the catalytic cycle. This effect avoids the formation of hydroxyl radicals that is observed with mononuclear Mn-based complexes. Experiments using a stroke model in rats showed that the complex could reduce nerve-cell damage and improve neurological outcomes.
- Tri-Manganese(III) Salen-Based Cryptands: A Metal Cooperative Antioxidant Strategy that Overcomes Ischemic Stroke Damage In Vivo,
Yingying Ning, Yan Huo, Haozong Xue, Yujing Du, Yuhang Yao, Adam C. Sedgwick, Hengyu Lin, Cuicui Li, Shang-Da Jiang, Bing-Wu Wang, Song Gao, Lei Kang, Jonathan L. Sessler, Jun-Long Zhang,
J. Am. Chem. Soc. 2020.
https://doi.org/10.1021/jacs.0c03805