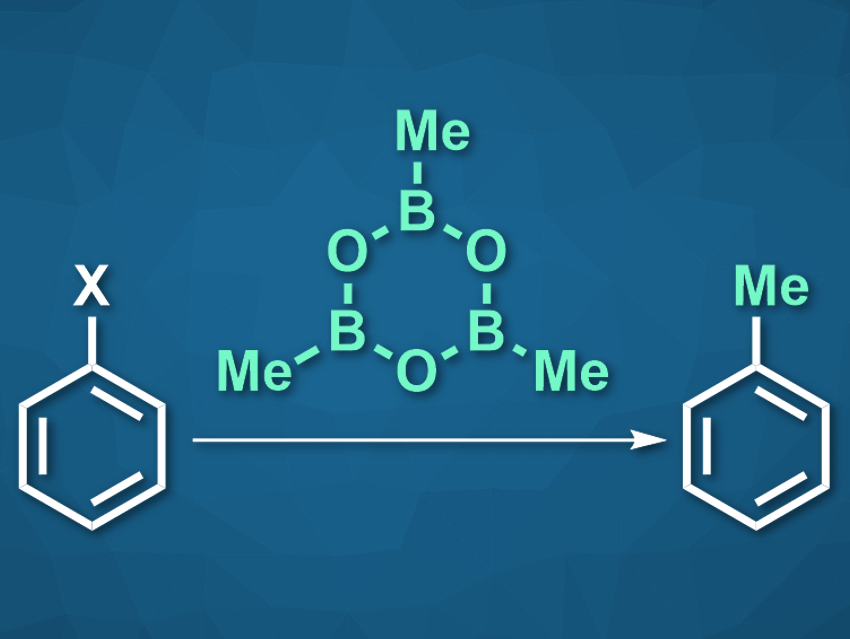
Methylation reactions can change the physical properties and biological activity of organic molecules. This can be particularly useful in pharmaceutical chemistry. Aryl electrophiles, for example, can be methylated via a transition-metal-catalyzed cross-coupling reaction with a nucleophilic reagent. However, this type of methylation is often limited to aryl halides such as bromides and iodides as the electrophilic reaction partners. Other aryl electrophiles, such as arenes with cyanide-, nitro-, amide-, or ester groups, can be challenging to methylate.
Boya Feng, Yudong Yang, and Jingsong You, Sichuan University, Chengdu, China, have used trimethylboroxine (TMB, pictured) as a low-cost methylating reagent for these more challenging aryl electrophiles, and thus, expanded the scope of transition-metal-catalyzed aryl methylation. The team used Pd(acac)2 (acac = acetylacetonate) as the catalyst, together with BrettPhos (2-(dicyclohexylphosphino)3,6-dimethoxy-2′,4′,6′-triisopropyl-1,1′-biphenyl) as a ligand, to convert a variety of, e.g., nitroarenes, to the corresponding methylated compounds.
The ligands and even the transition metal can be varied to optimize the reaction with different aryl substrates. Benzoic acids, for example, can be efficiently methylated using Pd(OAc)2 and dcype (dcype = 1,2-bis(dicyclohexylphosphino)ethane), and aryl fluorides react well in the presence of Ni(cod)2 (cod = 1,5-cyclooctadiene) and dcype. TMB can be universally used as the methylating agent in all these reactions. According to the researchers, the reaction could be useful in drug and materials development.
- Methylation Platform of Unconventional Inert Aryl Electrophiles: Trimethylboroxine as a Universal Methylating Reagent,
Boya Feng, Yudong Yang, Jingsong You,
Chem. Sci. 2020.
https://doi.org/10.1039/d0sc01641a