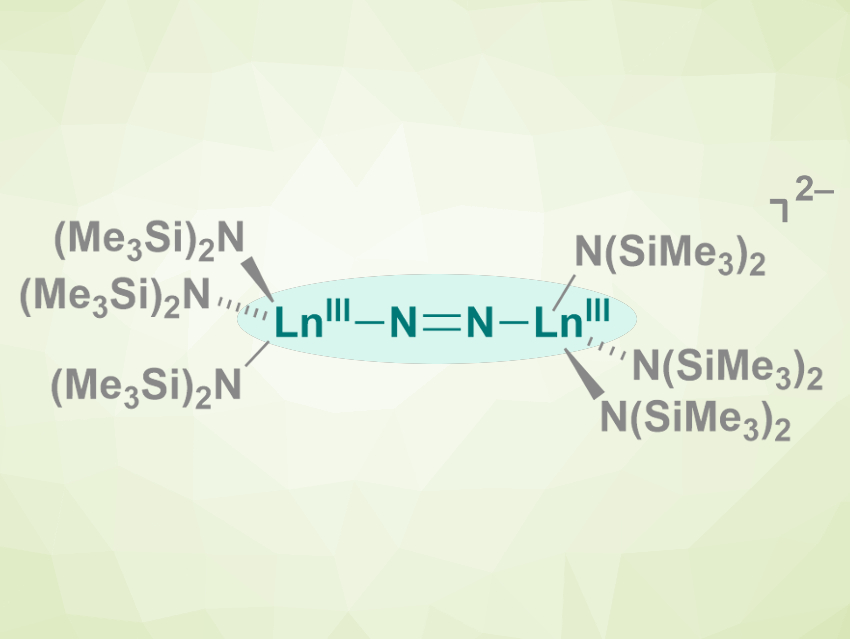
There are dozens of known reduced-dinitrogen complexes with rare-earth metals, i.e., complexes of the type LnLn2N2 (L= solvent molecules, amides, or anionic organic ligands, Ln = rare-earth metal). In all complexes of this type that had been reported so far, the N22– anion is coordinated in a side-on manner to the metal atoms.
Filipp Furche, William J. Evans, and colleagues, University of California, Irvine, USA, have synthesized the first end-on lanthanide Ln–N═N–Ln-type species (pictured, Ln = Tb, Gd). The team reacted the salts [K(crypt)][Ln(N(SiMe3)2)3] or [K(18c6)2][Ln(N(SiMe3)2)3] (crypt = 2.2.2-cryptand, 18c6 = 18-crown-6) with dinitrogen at –35 °C in diethyl ether. The resulting crystals were characterized using X-ray diffraction.
The team found that Tb formed a complex anion with an end-on coordinated reduced dinitrogen (pictured). Gd, in contrast, formed a mixture of complexes with both side-on and end-on reduced-dinitrogen ligands. The team attributes this difference to the similar stability of the side-on and end-on Gd complexes, which was confirmed by density functional theory (DFT) calculations. The researchers point out that small changes in the size of the lanthanide, such as between Tb and Gd, can cause changes in the outcome of this type of reaction.
- Formation of the End-on Bound Lanthanide Dinitrogen Complexes [(R2N)3Ln–N═N–Ln(NR2)3]2– from Divalent [(R2N)3Ln]1– Salts (R = SiMe3),
Austin J. Ryan, Sree Ganesh Balasubramani, Joseph W. Ziller, Filipp Furche, William J. Evans,
J. Am. Chem. Soc. 2020.
https://doi.org/10.1021/jacs.0c01021