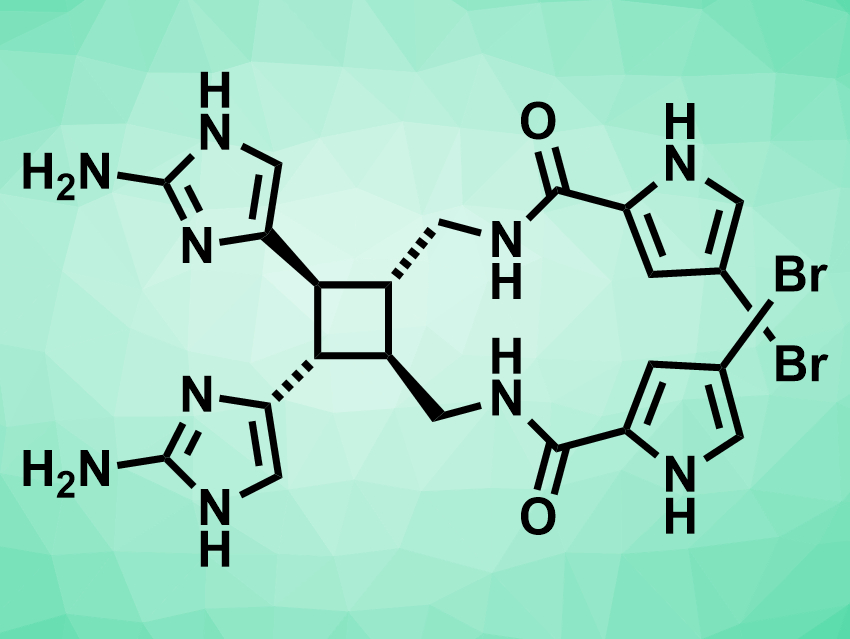
Sceptrin (pictured above) is a natural product that was first isolated from a marine sponge. It is bioactive and could have an antibiotic effect. Its biosynthesis is thought to be based on the [2+2] dimerization of hymenidin (pictured below). However, this reaction had not been achieved in the lab so far. Existing total syntheses of sceptrin require 11–25 steps and do not include this type of dimerization.
Long V. Nguyen and Timothy F. Jamison, Massachusetts Institute of Technology (MIT), Cambridge, USA, have performed a total synthesis of racemic sceptrin in four steps from commercially available compounds—the shortest synthesis reported to date. The team first built an imidazopyrimidine-containing substitute for hymenidin from N-(tert-butoxycarbonyl)-propargylamine and 3-bromoimidazopyrimidine. This intermediate was then dimerized under irradiation with blue LEDs in the presence of the iridium photocatalyst [Ir(dF(CF3)ppy)2(dtbbpy)]PF6 (dF(CF3)ppy = 3,5-difluoro-2-[5-(trifluoromethyl)-2-pyridinyl]phenyl, dtbbpy = di-tert-butylbipyridine) to form the cyclobutane core.
 After this key dimerization step, the 3-bromopyrrole groups (pictured above on the right-hand side of the molecule) were installed via the reaction of the dimerized intermediate with a trichloroacetylbromopyrrole. The final product was then obtained by the addition of hydrazine, which converts the imidazopyrimidine groups to the desired aminoimidazole groups (pictured above on the left-hand side of the molecule). Using this approach, the team prepared multiple grams of (±)-sceptrin, which should enable studies of its biological activity.
After this key dimerization step, the 3-bromopyrrole groups (pictured above on the right-hand side of the molecule) were installed via the reaction of the dimerized intermediate with a trichloroacetylbromopyrrole. The final product was then obtained by the addition of hydrazine, which converts the imidazopyrimidine groups to the desired aminoimidazole groups (pictured above on the left-hand side of the molecule). Using this approach, the team prepared multiple grams of (±)-sceptrin, which should enable studies of its biological activity.
- Total Synthesis of (±)-Sceptrin,
Long V. Nguyen, Timothy F. Jamison,
Org. Lett. 2020.
https://doi.org/10.1021/acs.orglett.0c01381