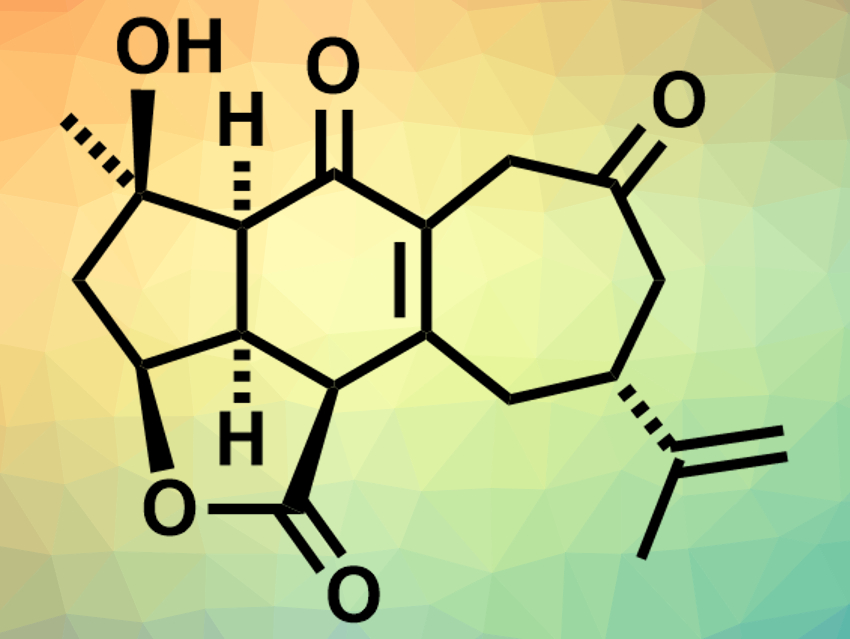
Scabrolide A (pictured) is a polycyclic marine natural product and part of a family of isomeric compounds that were isolated from soft corals. These compounds (polycyclic furanobutenolide-derived norcembranoid diterpenoids) have complex structures with synthetically challenging oxygenation patterns and have been difficult to access by total synthesis so far. Scabrolide A could have potential as an anti-inflammatory agent.
Brian M. Stoltz, California Institute of Technology, Pasadena, USA, and colleagues have performed the first total synthesis of (−)-scabrolide A. The team started from (R)-linalool and (R)-carvone, which were transformed to a dihydroxyvinylcyclopentene and an ynoic acid, respectively. These fragments were reacted to give an ester containing all 19 of the carbon atoms of scabrolide A. This ester was converted to a tricyclic intermediate via a Diels–Alder cycloaddition. This intermediate was then converted to an enone via a vanadium-catalyzed epoxidation, a titanium-catalyzed epoxide opening, and an oxidation with 2-iodoxybenzoic acid (IBX). The resulting enone was transformed into the desired product with its cycloheptenone ring via an enone–olefin cycloaddition and an oxidative fragmentation/recombination.
The physical and spectroscopic data of the product are in agreement with those reported for natural scabrolide A. The team also confirmed the structure using X-ray diffraction. According to the researchers, this is the first example of a total synthesis of any member of the polycyclic furanobutenolide-derived norcembranoid diterpenoid family.
- The Total Synthesis of (−)-Scabrolide A,
Nicholas J. Hafeman, Steven A. Loskot, Christopher E. Reimann, Beau P. Pritchett, Scott C. Virgil, Brian M. Stoltz,
J. Am. Chem. Soc. 2020.
https://doi.org/10.1021/jacs.0c02513