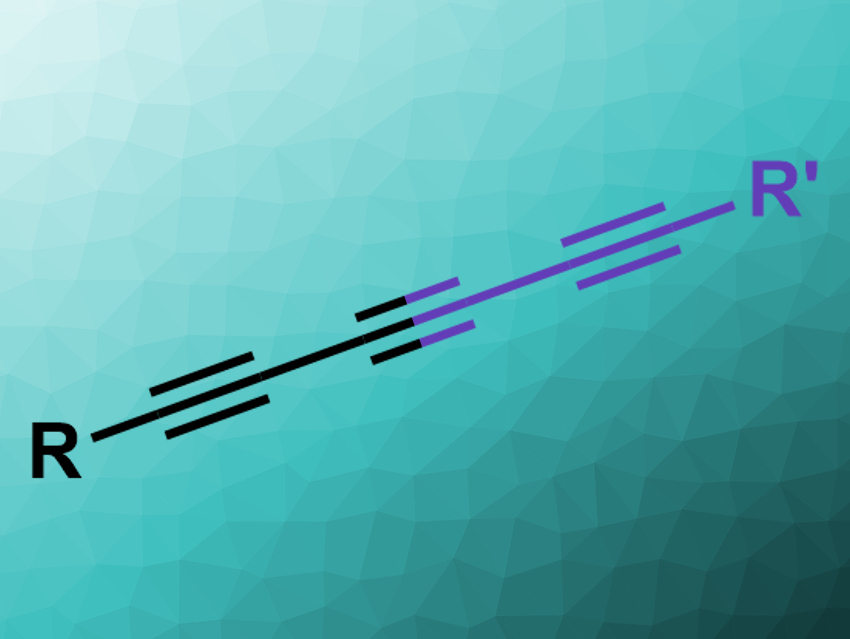
Polyynes, or compounds with multiple conjugated C≡C triple bonds, are often found in bioactive natural products. They can, for example, be synthesized via cross-coupling or metathesis reactions. However, the selective formation of conjugated triynes (pictured) can be challenging.
Yann Trolez, Marc Mauduit, and colleagues, Ecole Nationale Supérieure de Chimie de Rennes, France, have achieved the first synthesis of conjugated triynes by molybdenum-catalyzed alkyne metathesis. The team first synthesized different diynes, each with one methyl substituent and one more sterically demanding substituent (e.g., a triphenylmethyl group). These diynes were then reacted in the presence of the molybdenum catalyst [Mo(≡C–C6H4OMe)(OSiPh3)3] and 4 Å/5 Å molecular sieves (MS). The reaction was performed in toluene at 40 °C.
The metathesis reaction converts two equivalents of diyne into one equivalent of the desired triyne and one equivalent of 2-butyne, i.e., Me–C≡C–Me. Both symmetrically substituted (R =R’) and unsymmetrical (R ≠R’) triynes can be synthesized. The team found that the sterically demanding substituents are required to achieve a high selectivity for the formation of the desired triynes and prevent the formation of diynes. They reached triyne:diyne ratios up to 95:5.
- Expedient Synthesis of Conjugated Triynes via Alkyne Metathesis,
Idriss Curbet, Sophie Colombel-Rouen, Romane Manguin, Anthony Clermont, Alexandre Quelhas, Daniel S. Müller, Thierry Roisnel, Olivier Basle, Yann Trolez, Marc Mauduit,
Chem. Sci. 2020.
https://doi.org/10.1039/d0sc01124j