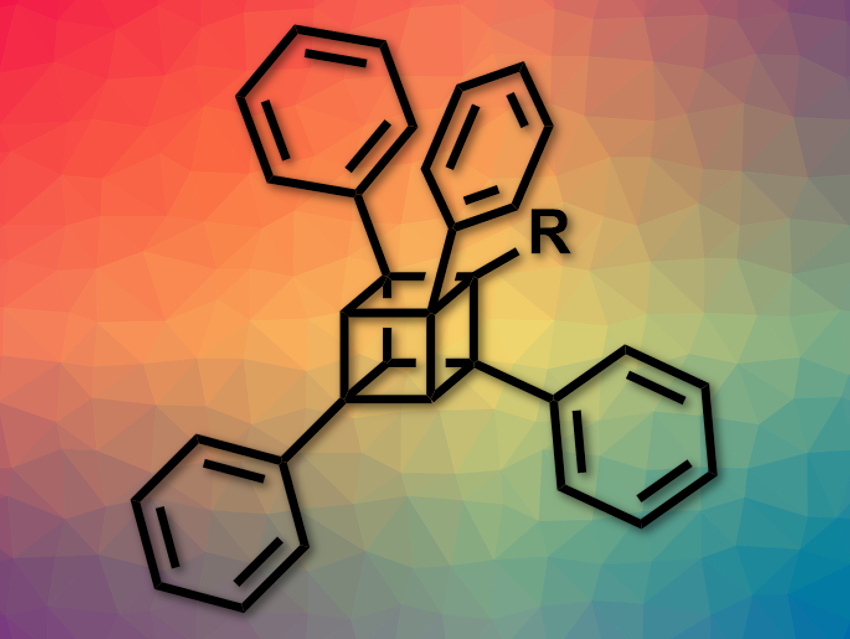
Cubane (C8H8) is a cube-shaped, strained hydrocarbon. Its derivatives can, for example, be used in bioactive compounds, metal–organic frameworks, or high-energy materials. However, the synthesis of many cubane derivatives is challenging. Most often, 1- and/or 4-substituted cubanes have been synthesized because they can be made from commercially available 1,4-cubanedicarboxylic acid.
Akiko Yagi, Kenichiro Itami, Nagoya University, Japan, and colleagues have developed a process for the regioselective arylation of amidocubanes. The approach allows the regioselective, late-stage installation of different aryl groups and the preparation of mono-, di-, tri -, or tetraarylated cubanes.
The team started from a 1-(N,N-diisopropylamido)-4-cyanocubane, which was first reacted with lithium tetramethylpiperidide (LiTMP) and ZntBu2, and then with an aryl iodide in the presence of palladium catalyst system. This results in the formation of monoaryl cubanes. The process is based on a directed ortho-C–H metalation next to the amido group, followed by a palladium-catalyzed cross-coupling to install the aryl in the ortho-position.
The reaction can be repeated two more times to install different aryl substituents in all three possible ortho-positions next to the amido group. A fourth aryl group can be introduced in place of the original cyano group at the 4-position by hydrolysis and subsequent decarboxyphenylation of the CN group.
- Programmable Synthesis of Multiply Arylated Cubanes through C-H Metalation and Arylation,
Kenichiro Itami, Ryo Okude, Genki Mori, Akiko Yagi,
Chem. Sci. 2020.
https://doi.org/10.1039/d0sc01909g