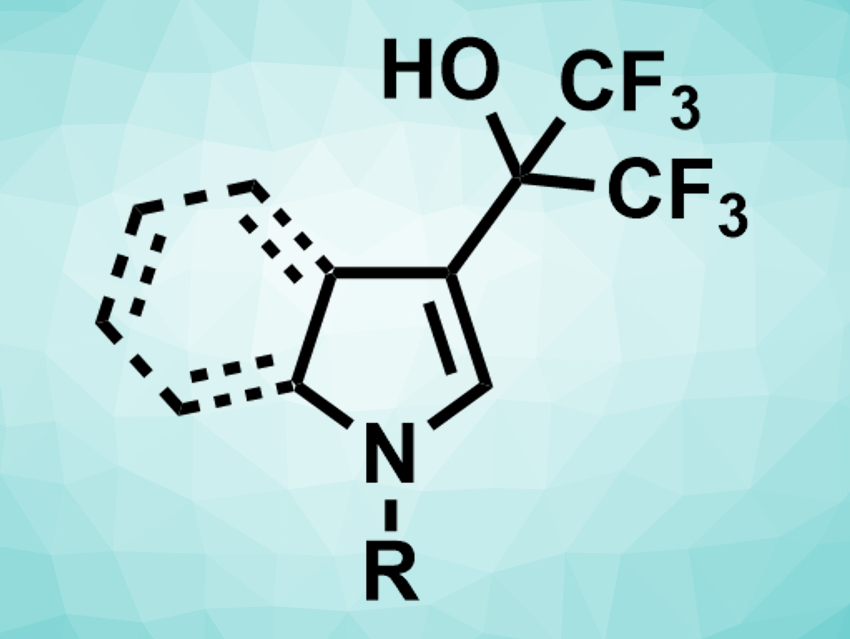
Organofluorine compounds are often used, e.g., in medicinal or agricultural chemistry. While there are several ways to introduce fluoroalkyl groups into organic compounds, using fluoroalcohols in direct coupling reactions has not been well explored. This is despite of the fact that fluoroalcohols are used as stable, easy-to-handle, and low-cost solvents.
Wangbin Sun, Yaojia Jiang, Nanjing Tech University, China, and colleagues have developed a copper-catalyzed direct C(sp2)–H/C(sp3)–H coupling reaction of aza-aromatic rings with fluoroalcohols (example product pictured). This reaction provides access to hydroxyfluoroalkylated aniline, pyrrole, and indole derivatives. The team used Cu(OAc)2 as a catalyst, 4,4′-di-tert-butyl-2,2′-bipyridine (DTBBPY) as a ligand, and hexafluoroisopropanol (HFIP) as both solvent and reactant. The reaction was performed at 90 °C.
The desired hydroxyfluoroalkylated products were obtained in moderate to excellent yields. Substrates such as methyl-substituted indoles were converted smoothly, while reactants with electron-withdrawing substituents gave lower yields. For the reaction mechanism, the researchers propose a catalytic electrophilic substitution with hexafluoroacetone as the key intermediate.
- Copper-Catalyzed Functionalization of Aza-Aromatic Rings with Fluoroalcohols via Direct C(sp2)–H/C(sp3)–H Coupling Reactions,
Jie Chen, Meng Li, Jinli Zhang, Wangbin Sun, Yaojia Jiang,
Org. Lett. 2020.
https://doi.org/10.1021/acs.orglett.0c00797