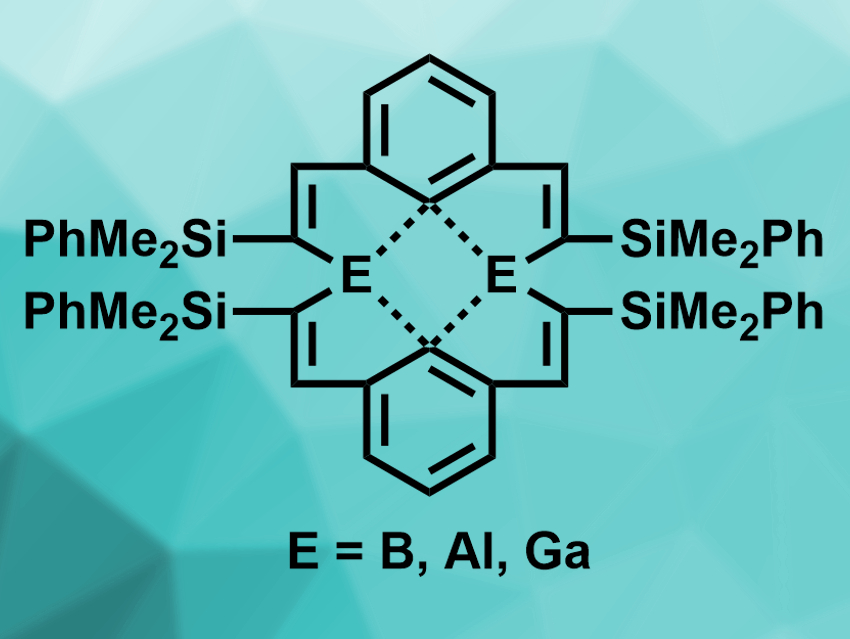
1,3-Bis(silylethynyl)benzenes of the type C6H4(C≡C–SiMe2R)2 (R = Me,Ph) can be transformed into unusual trilithium derivatives. These trilithium compounds feature three anionic carbon atoms in a single molecule, one at the central aromatic ring and two at the vinylic carbon atoms. These triple anions could be useful chelating ligands.
Werner Uhl, University of Münster, Germany, and colleagues have reacted the phenyl-substituted derivative of these trilithium compounds with the group 3 halides BCl3, AlBr3, or GaCl3. They found that unprecedented dinuclear heptacyclic compounds (pictured) were formed, with yields of 12 % for B, 71 % for Al, and 58 % for Ga. The compounds were characterized using 1H and 13C NMR spectroscopy, as well as X-ray crystallography.
The researchers found that the two chelating ligands in the products form a helical shape, resulting in a distorted tetrahedral coordination sphere at the group 3 atoms. Structural data and the results of density functional theory (DFT) calculations indicate that the central aryl carbon atoms form two three-center–two-electron (3c–2e) bonds with the group 3 atoms. According to the team, the triple-anionic ligands could also be useful in transition-metal or rare-earth chemistry.
- Tridentate Ligand with Three Carbanions as Donor Atoms: Formation of Dinuclear, Heptacyclic Complexes of Boron, Aluminum, or Gallium with B–C–B, Al–C–Al, or Ga–C–Ga Three-Center–Two-Electron Bonds,
Alexander Brand, Alexander Hepp, Ernst-Ulrich Würthwein, Werner Uhl,
Inorg. Chem. 2020.
https://doi.org/10.1021/acs.inorgchem.0c00254