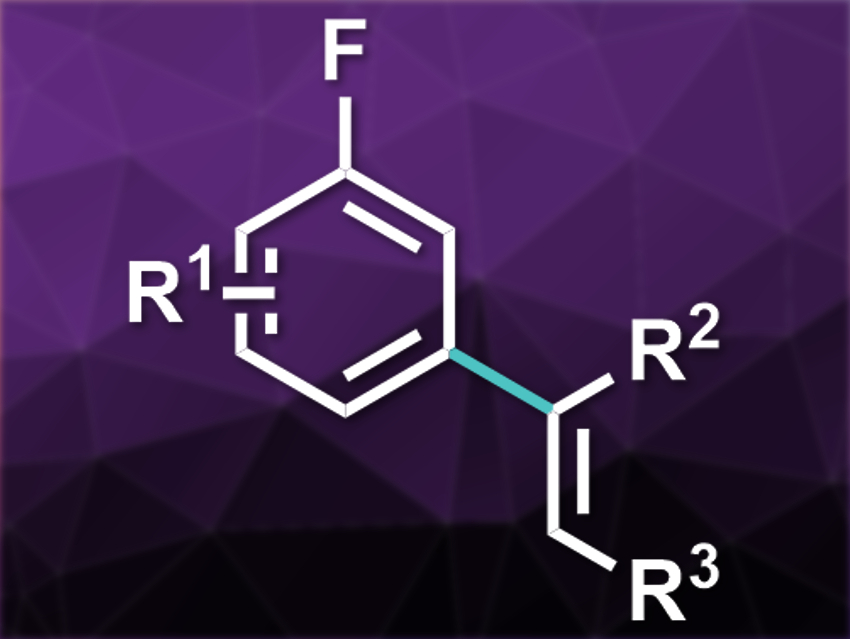
Aryl olefins are useful intermediates and products in organic synthesis. There is a variety of methods for the ortho-olefination of arenes, but few approaches for the meta-selective olefination. Existing methods for the meta-selective reaction require directing groups that cannot be installed on fluoro-substituted arenes. However, fluoroarenes are common, e.g., in pharmaceutical chemistry.
Igor Larrosa and colleagues, University of Manchester, UK, have developed a meta-selective olefination of fluoroarenes that uses CO2 as a traceless, easy-to-install directing group. The olefination can be performed in a one-pot process. The team first used a lithiation/carboxylation protocol with sec-butyllithium and gaseous CO2 to install the carboxylate directing group at the ortho-position. The olefination was then performed with a range of alkynes, using [Ru(C6Me6)(OAc)2] as a catalyst and 1,2-dichloroethane (DCE) as the solvent.
The desired products were obtained in good to excellent yields and with complete meta-regioselectivity. According to the researchers, this is the first example of a method for the meta-selective olefination of fluoroarenes.
- meta-Selective olefination of fluoroarenes with alkynes using CO2 as a traceless directing group,
Andrew Spencer, Rishi Korde, Marc Font, Igor Larrosa,
Chem. Sci. 2020.
https://doi.org/10.1039/d0sc01138j



![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)