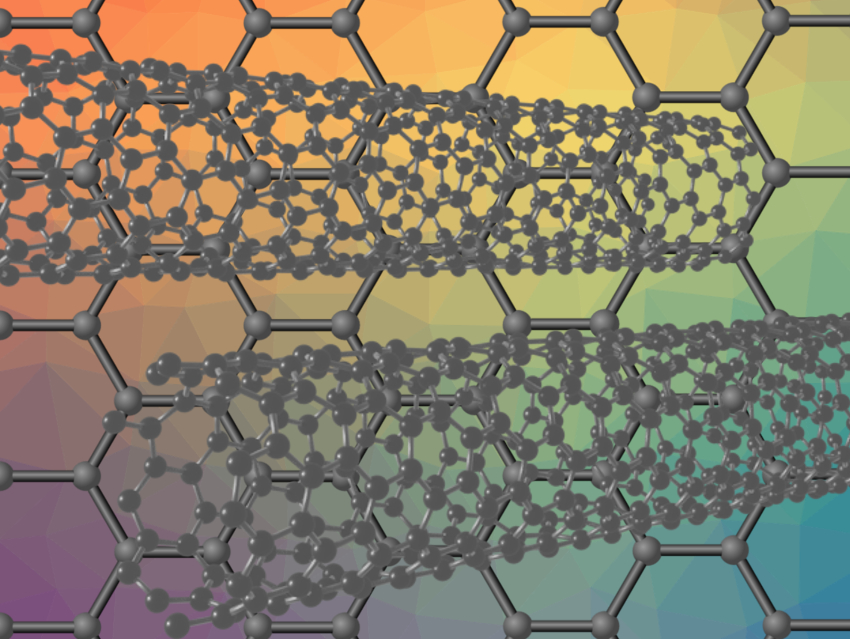
The oxygen reduction reaction (ORR) and the oxygen evolution reaction (OER) are important for water splitting and in fuel cells. Good catalysts for these reactions are, thus, key for an environmentally friendly hydrogen economy. Often, such catalysts are based on expensive metals such as Pt, Ru, or Ir. Using doped carbon-based materials as catalysts could be a cost-effective alternative.
Mohammad Tavakkoli, Aalto University, Finland, and colleagues have developed a mesoporous graphene nanoflake (GF)/carbon nanotube (CNT) hybrid material doped with single atoms of N, Co, and Mo. The material was prepared via a one-step catalytic chemical vapor deposition method under a flow of H2 and CH4, using a powder of Mg0.99(Co1-xMox)0.01O (x ≈ 0.25) as the catalyst and acetonitrile as the nitrogen source.
The resulting hybrid material has a high electrocatalytic activity and stability for ORR and OER in alkaline solutions. The mesoporous structure improves the transfer of oxygen within the catalyst film and provides a large surface area. The team found that metal–carbon centers are the main active sites for the OER, while metal and nitrogen–carbon centers together act as active sites for the ORR. When the hybrid material is placed on a nickel support, the resulting catalyst has an even higher catalytic activity. According to the researchers, the Ni-supported catalyst has an electrocatalytic performance that is comparable to the best ORR/OER catalysts reported so far.
- Mesoporous Single-Atom-Doped Graphene‒Carbon Nanotube Hybrid: Synthesis and Tunable Electrocatalytic Activity for Oxygen Evolution and Reduction Reactions,
Mohammad Tavakkoli, Emmanuel Flahaut, Pekka Peljo, Jani Sainio, Fatemeh Davodi, Egor V. Lobiak, Kimmo Mustonen, Esko I. Kauppinen,
ACS Catal. 2020.
https://doi.org/10.1021/acscatal.0c00352