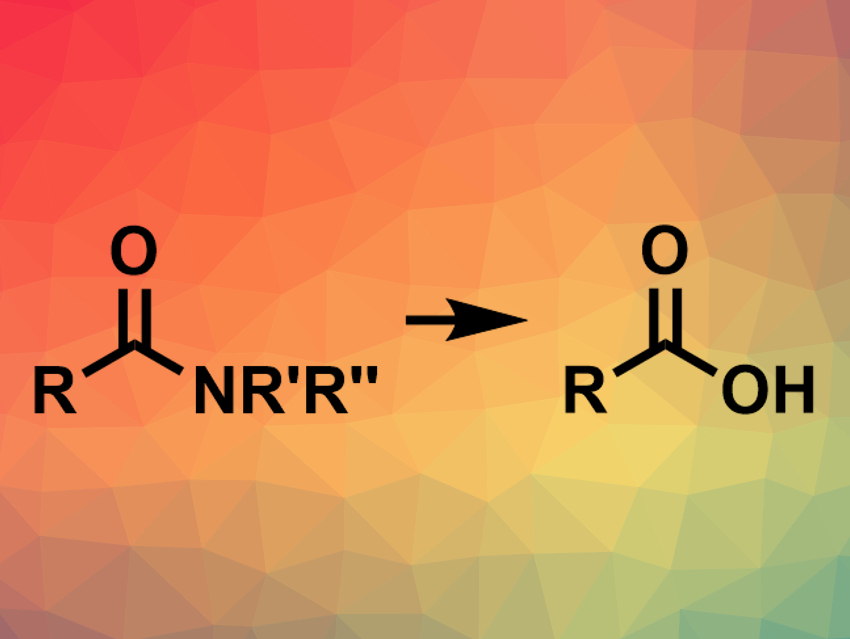
Amides are useful synthetic building blocks in a range of reactions catalyzed by palladium, rhodium, or nickel. They can, for example, be converted to esters, ketones, or other amides. However, the transformation of amides to carboxylic acids (pictured) via transition-metal-catalyzed C–N bond activation had not been reported so far.
Neil K. Garg, University of California, Los Angeles, USA, and colleagues have developed a reaction that converts amides to carboxylic acids using nickel catalysis. The reaction proceeds via an esterification with 2-(trimethylsilyl)ethanol catalyzed by nickel, followed by a fluoride-mediated deprotection that gives the desired carboxylic acid. This sequence is performed in a one-pot process. The team used Ni(cod)2 as the catalyst (cod = 1,5-cyclooctadiene), tetra-n-butylammonium fluoride (TBAF) as a fluoride source, and toluene as the solvent. The reaction was performed at 110 °C.
A range of amides with aryl groups and different substituents at nitrogen were converted to the corresponding carboxylic acids in moderate to excellent yields. The reaction is mild and selective, which allows its use on complex substrates.
- Nickel-Catalyzed Conversion of Amides to Carboxylic Acids,
Rachel R. Knapp, Ana S. Bulger, Neil K. Garg,
Org. Lett. 2020.
https://doi.org/10.1021/acs.orglett.0c00885