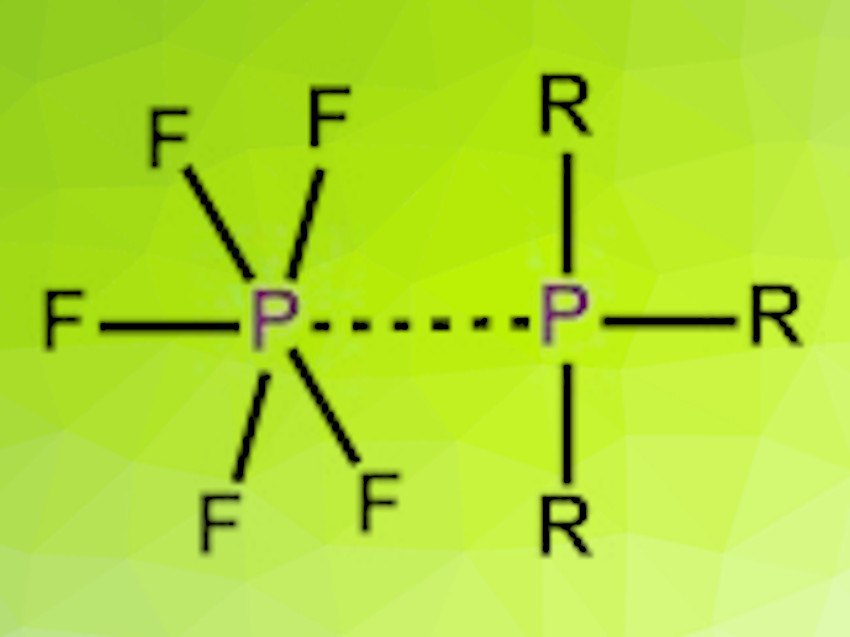
Phosphorus pentafluoride (PF5) acts as a Lewis acid and can form complexes with fluoride ions or neutral Lewis bases such as O- or N-donor ligands and solvents. For example, bidentate N-donor-ligands such as 2,2-bipyridine form ionic complexes of the structure [PF4(diimine)][PF6]. Less research attention has been given to PF5 complexes of ligands with heavier donor atoms.
Gillian Reid, University of Southampton, UK, and colleagues have synthesized a series of phosphine and arsine complexes of PF5 of the types [PF5(PR3)] and [PF4(o-C6H4(PR2)2)][PF6], as well as the unstable [PF5(AsMe3)]. The phosphine complexes were synthesized by bubbling PF5 gas through a solution of the respective phosphine in anhydrous n-hexane or dichloromethane. The complexes easily undergo hydrolysis but appear to be stable under dry nitrogen. They were analyzed by 1H-, 19F- and 31P-NMR spectroscopy and single-crystal X-ray diffraction.
The arsine complex was synthesized the same way and analyzed by low-temperature NMR spectroscopy at 183 K. The product was too unstable for further analysis, however. Overall, it was shown that phosphines can form complexes with PF5 and that diphosphines form ionic complexes similar to bidentate nitrogen ligands. Arsines, in contrast, displayed a lower affinity for PF5 and were not able to form stables complexes.
- Tertiary Phosphine and Arsine Complexes of Phosphorus Pentafluoride: Synthesis, Properties, and Electronic Structures,
John M. Dyke, James W. Emsley, Victoria K. Greenacre, William Levason, Francesco M. Monzittu, Gillian Reid, Giuseppina De Luca,
Inorg. Chem. 2020.
https://doi.org/10.1021/acs.inorgchem.9b03630